Abstract
Worksite smoking cessation programs offer accessibility of the target population, availability of occupational health support, and the potential for peer pressure and peer support. The purpose of this study was to identify the efficacy of the financial incentives given to various teams in the workplace. St. Paul's Hospital's employees were enrolled. Each team of employees consisted of smoking participants and non-smoking fellow workers from the same department. The financial incentive of 50000 won (about $45) was rewarded to the team for each successful participant-not to individual members-after the first week and then after one month. If the smokers in the team remained abstinent for a longer time period, the team was given an incentive of 100000 won for each successful participant after 3 and 6 months. A total 28 smoking participants and 6 teams were enrolled. Self-reported abstinence rates validated by urinary cotinine test at 3, 6, and 12 months after the initial cessation were 61%, 54%, and 50%, respectively. Smokers with high nicotine dependence scores or those who began participation 1 month after enrollment initiation had a lower abstinence rate at 3 months, but not at 6 and 12 months. Participants who succeeded at smoking cessation at 12 months were more likely to be older and have a longer smoking duration history. The financial incentives given to teams could be promising and effective to improve long-term rates of smoking cessation. This approach could use peer pressure and peer support in the workplace over a longer period.
Smoking is a leading cause of preventable death and contributes to approximately 438000 deaths each year in the United States.1 Current estimates show that the prevalence of active cigarette smoking in Korea is 26%, and the rate in the US is currently at 21%.2 Seventy percent of smokers report being interested in quitting, and 40% attempt to quit each year.3 However, only 2 to 3% of smokers succeed annually.4 Physician's advice can increase quitting by a further 1 to 3%,5 while behavioral support is likely to increase the chance of success by about 10 to 25%.6 Pharmacotherapy, including nicotine replacement therapy with varenicline and bupropion, has been shown to be effective for long term (≥6 months) quit rates by 15% to 30% in combination with behavioral support.7,8,9 Moreover, financial incentives given to employees showed about 15% abstinence rate.4
The workplace offers a promising venue for encouraging smoking cessation. The workplace as a setting for smoking cessation research and intervention has several advantages, including the accessibility of the target population, the availability of occupational health support and the potential for peer pressure and peer support.10,11 Moreover, financial incentives and competitions for smoking cessation represent one intervention option by worksite health promotion programs.4,11,12 These interventions are postulated to work thro-ugh one or more of the following pathways: 1) increasing or improving motivation to quit; 2) increasing or improving action to quit; and 3) increasing or improving maintenance of efforts to quit.12 Workers who quit smoking could contribute to reductions in tobacco-related morbidity and mortality.12 To our best knowledge, there has been no report to investigate the abstinence rate of team-based intervention in the workplace.
The purpose of this study was to identify the efficacy of the financial incentives given to teams in improving long-term rates of smoking cessation among employees.
We recruited study participants from health care workers at St. Paul's Hospital during January to June 2011. Employees were eligible to participate if they were at least 18 years of age and if they reported that they were currently smoking five or more cigarettes per day for the prior 12 months and were willing to attempt to stop smoking permanently. Potential participants were identified with the use of a survey that asked employees about their smoking habits and their willingness to be contacted by researchers in a smoking-cessation study. Employees were not included in the study if they were planning to leave St. Paul's Hospital within the next 12 months.
We collected information on age, body mass index, baseline smoking history, interest in quitting, spirometric indices and pretreatment level of nicotine dependence score on the Fagerström Test for Nicotine Dependence (FTND)13 (which has a range of 0 to 10, with higher scores indicating more nicotine dependence); a score of 6 or greater was classified as high nicotine dependency.
All participants provided written informed consent. The study protocol was approved by the Institutional Review Board at St. Paul's Hospital, The Catholic University of Korea. This study was registered with ClinicalTrials.gov, number NCT01323725.
Each team consisted of smoking participants and non-smoking fellow workers from the same department. All team members were informed that the teams they belonged to would receive financial incentives based on their team efforts at smoking cessation, which is to engage social networks to provide social support and pressure for the quit attempt and abstinence. All smoking participants were given usual smoking cessation counseling and 3-month coverage of varenicline, a selective nicotine receptor partial agonist. In addition, nicotine replacement therapy (NRT) was permitted.
Smoking participants were contacted at 1, 2, 4, and 8 weeks and 3, 6, and 12 months after enrollment and asked whether they had stopped smoking. Participants who reported abstinence (not more than five cigarettes from the start of the abstinence period) were examined by urine cotinine test every three months after enrollment. The financial incentive of 50000 won (about $45) was rewarded to the team for each successful participant after the first week and then after one month, but not to individual smokers. If the smokers in the team remained abstinent for a longer time period, the team was given team incentive of 100000 won for each successful smoker at 3 and 6 months.
The primary endpoint was the participant's continuous smoking abstinence at 6 months. Self-reported abstinence allowing up to five cigarettes in total was validated by a negative urinary cotinine test.14 The secondary endpoints included rates of smoking abstinence verified by urine cotinine tests at 3 and 12 months after study enrollment.
All data are reported as means±standard error of the mean or frequencies (%). An analysis was performed between the success and failure group using the t-test or the Mann-Whitney U test for continuous variables and the chi-squared test or Fisher's exact test for categorical variables. Tests for trends were estimated by a linear-by-linear association. All tests were two-sided and a p<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software version 15.0.0 for Windows (SPSS Inc., Chicago, IL, USA).
Table 1 shows demographic characteristics of total 28 smoking participants enrolled in the study. All participants were male and the mean age was 40±9.02 years. The total number of team was 6, and 3 to 8 smoking participants were included in each team. Among them, fourteen who were not planning to quit smoking immediately participated 1 month after recruitment. Nineteen participants (68%) took the 3- month coverage of varenicline as directed while they were being abstinent, and 3 participants used additional NRT, including nicotine patch or electronic cigarette.
Self-reported abstinence rates validated by urinary cotinine test at 3, 6, and 12 months were 61%, 54%, and 50%, respectively. Table 2 gives the differences in demographics at 3, 6, and 12 months between the success and the failure groups. FTND scores were significantly higher in the failure group at 3 months (p<0.05), but not at 6 and 12 months. Smokers who participated within 1 month of enrollment had a higher abstinence rate at 3 months than those who participated 1 month after enrollment initiation (p<0.01). Participants who succeeded in smoking cessation at 12 months were more likely to be older and have longer smoking duration (p<0.01). However, there were no differences in body mass indices and spirometric indices between the success and the failure groups at 3, 6, and 12 months. Linear association by testing for a trend was not found between the number of smoking participants enrolled and the abstinence rate (data not shown).
In this study of team-based financial incentives for smoking cessation for employees, the rates of abstinence at 3, 6 and 12 months were 61%, 54%, and 50%, respectively. These interventions for smoking cessation could be a promising and effective option for worksite health promotion programs and our data demonstrated favorable abstinence rates through 12 months.
Worksite-based financial incentives and competitions are known to be effective in reducing tobacco use.4,11,12 A variety of rewards have been used for smoking cessation, including cash payments, salary bonuses and promotional items.11 According to a large randomized controlled trial, financial incentives given to employees resulted in higher abstinence rates at 9 or 12 months after enrollment in the incentives group compared to the control group (14.7% vs. 5.0%).4 There were two trials, which rewarded the worksite as a whole for employees' performance with cash payment.15,16 However, individual prizes for successful participants were also present in those trials. Nevertheless, clinical trials examining team-based financial incentives are lacking. In the present study, although incentives were given to the teams until 6 months, high abstinence rates were maintained for 12 months. These results suggest that team-based incentives in the workplace encouraged participants' motivation for smoking cessation, moreover, these interventions potentiated additional support to stay abstinent through peer-pressure and peer-support.
Financial incentives for participation in studies ranged from $10 to $750 for extended tobacco-use abstinence.4,17 Lottery-chance rewards ranged in size from $4018 to $500,17 and one study offered entrance in a $2500 lottery as part of the team reward.19 In the present study, a total 300000 won per person was given to the team for continuous smoking abstinence at 6 months, which was equal to $270 at that time. Studies comparing the magnitude of financial incentives for tobacco-use abstinence are lacking, and further study on the efficacy of a variety of different rewards as a tool to promote abstinence is needed.
Many pharmacologic treatments, including NRT, bupropion hydrochloride, and varenicline tartrate have been shown to be significantly effective.20 Varenicline, a selective α4β2 nicotinic receptor partial agonist specially developed for smoking cessation, is the only treatment demonstrating superior effects over other options.8,20 According to Nides, et al.,8 continuous abstinence rate from week 4 to week 52 in smokers prescribed 1.0 mg of varenicline twice daily was 14.4%. In our study protocol, all participants were given 3-month coverage of varenicline and NRTs were also permitted. Although these pharmacologic treatments might partially promote abstinence rates, our results were far superior to previously reported results with pharmacologic treatments, highlighting the effectiveness of team-based financial incentives for smoking cessation.
Traditionally, the transtheoretical model assumes that a smoker goes through a series of steps before quitting successfully: precontemplation (no thought of quitting), contemplation (thinking about quitting), preparation (planning to quit in the next 30 days), action (quitting successfully for up to six months) and maintenance (no smoking for more than six months).21 Although the statistical differences were not maintained over a prolonged period, smokers who participated 1 month after enrollment initiation or those who had higher nicotine dependence scores showed a lower abstinence rate at 3 months. These results indicate that smokers enrolled within 1 month were more likely to be in the contemplation-preparation stages in our study and their abstinence rates were more favorable in the short term. In addition, stepped smoking cessation intervention based on the transtheoretical model would be required for smokers with high FTND scores in the precontemplation stage to be motivated into the preparation-action stage.13,21,22,23
Tillgren, et al.24 showed that daily consumption of cigarettes, years spent smoking and age were associated with successful quitting. According to the from a Korean adult smokers' survey,25 as age increased, the proportion of smokers in the contemplation and preparation stages declined, while the percentage in the precontemplation stage increased. However, the maintenance of abstinence increased over longer periods as age increased.26 In our study, participants who were older and had a longer smoking history were more likely to maintain abstinence at 12 months. We postulate that these participants were likely to be more concerned about their health problems and these interventions could help provide motivation to change from the action to maintenance stage.
Our study has several limitations. First, the number of participants was relatively small. The smoking rate in our worksite was relatively low, accounting for about 60 people among around 600 employees. Thus, we believe that the enrolled participants were representative of the group of employees willing to quit smoking. Second, we did not perform a randomized trial comparing incentives given to teams with those given to individual participants. Third, health care workers might have more knowledge regarding to smoking's harmful effects and smoking-related respiratory system disease than the general public, which might result in positive attitude regarding the cessation of smoking.27
In conclusion, team-based financial incentives resulted in a high abstinence rate, which was maintained over a 12- months period. These results suggest that the financial incentives given to teams could make the use of potential for peer pressure and peer support over a longer period. More short-term intensive management is needed for members who participate later in the intervention or have higher nicotine dependence scores. Interestingly, participants who were older or had a longer smoking history were more likely to stay abstinent for a prolonged period. Further large-scale and controlled trials examining the potential for team-based interventions in improving long-term rates of smoking cessation within workplace are needed.
Figures and Tables
Table 1
Characteristics of the Study Participants

Table 2
Differences in Demographics at 3, 6, and 12 Months between the Success and Failure Groups
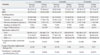
FTND, Fagerström test for nicotine dependence; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75%, forced expiratory flow between 25% and 75% of functional vital capacity; BMI, body mass index.
Success for smoking cessation indicates the participant's continuous self-reported abstinence (allowing up to five cigarettes in total) which was validated by a negative cotinine test on urine.
*p<0.05.
†p<0.01 for comparison between the success and the failure group for smoking cessation.
ACKNOWLEDGEMENTS
The authors thank Sun Hee Gang, an RN in the outpatient department of pulmonology, St. Paul's Hospital, The Catholic University of Korea for data collection and management
This work was supported by St. Paul's Hospital Research Program through The Catholic University of Korea.
References
1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004; 291:1238–1245.

2. Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med. 2012; 106:319–328.

3. Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: an effectiveness study. Prev Med. 2004; 38:412–420.

4. Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009; 360:699–709.

5. Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013; 5:CD000165.

6. Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012; 12:CD009670.

7. Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med. 2004; 140:426–433.

8. Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006; 166:1561–1568.

10. Smedslund G, Fisher KJ, Boles SM, Lichtenstein E. The effectiveness of workplace smoking cessation programmes: a meta-analysis of recent studies. Tob Control. 2004; 13:197–204.

11. Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev. 2011; CD004307.

12. Leeks KD, Hopkins DP, Soler RE, Aten A, Chattopadhyay SK;. Worksite-based incentives and competitions to reduce tobacco use. A systematic review. Am J Prev Med. 2010; 38:2 Suppl. S263–S274.
13. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrm KO. The Fagerstrm Test for Nicotine Dependence: a revision of the Fagerstrm Tolerance Questionnaire. Br J Addict. 1991; 86:1119–1127.

14. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005; 100:299–303.

15. Klesges RC, Glasgow RE, Klesges LM, Morray K, Quale R. Competition and relapse prevention training in worksite smoking modification. Health Educ Res. 1987; 2:5–14.

16. Hessol NA. Worksite smoking modification competitions: long-term vs short-term success. Am J Public Health. 1986; 76:819–820.

17. Hennrikus DJ, Jeffery RW, Lando HA, Murray DM, Brelje K, Davidann B, et al. The SUCCESS project: the effect of program format and incentives on participation and cessation in worksite smoking cessation programs. Am J Public Health. 2002; 92:274–279.

18. Gomel M, Oldenburg B, Simpson JM, Owen N. Work-site cardiovascular risk reduction: a randomized trial of health risk assessment, education, counseling, and incentives. Am J Public Health. 1993; 83:1231–1238.

19. Koffman DM, Lee JW, Hopp JW, Emont SL. The impact of including incentives and competition in a workplace smoking cessation program on quit rates. Am J Health Promot. 1998; 13:105–111.

20. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012; 44:588–597.

21. Cahill K, Lancaster T, Green N. Stage-based interventions for smoking cessation. Cochrane Database Syst Rev. 2010; CD004492.

22. Cabezas C, Advani M, Puente D, Rodriguez-Blanco T, Martin C. ISTAPS Study Group. Effectiveness of a stepped primary care smoking cessation intervention: cluster randomized clinical trial (ISTAPS study). Addiction. 2011; 106:1696–1706.

23. Fagerstrm K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrm Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012; 14:1467–1473.
24. Tillgren P, Haglund BJ, Lundberg M, Romelsj A. The sociodemographic pattern of tobacco cessation in the 1980s: results from a panel study of living condition surveys in Sweden. J Epidemiol Community Health. 1996; 50:625–630.

25. Jhun HJ, Seo HG. The stages of change in smoking cessation in a representative sample of Korean adult smokers. J Korean Med Sci. 2006; 21:843–848.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download