Abstract
Purpose
There are currently no consistently effective treatments for the excessive collagen produced by keloid fibroblasts. Previously, we reported that heat shock protein 70 (Hsp70) is up-regulated in keloid fibroblasts and keloid tissue. We, therefore, investigated whether Hsp70 is related to excessive collagen production in keloid fibroblasts.
Materials and Methods
We inhibited Hsp70 in keloid fibroblasts by RNA interference and examined the resulting collagen expression. Thus, we selected small interfering RNAs (siRNAs) specific for human Hsp70, transfected them into keloid fibroblasts, and evaluated the resulting phenotypes and protein production using real-time polymerase chain reaction (PCR), Western blot, and a collagen assay.
Keloids are characterized by an excessive accumulation of extracellular matrix, especially collagen, in an abnormal woundhealing process.1 Keloids cause cosmetic disfigurement, because they expand beyond the wound boundary and grow continuously, causing subjective symptoms such as itching and tingling sensations which disturb the patient's quality of life. The exact mechanism of keloid formation is not known; previous studies have, however, demonstrated the excessive deposition of collagen in the dermis.2 There are currently no consistently effective treatments, although several different approaches have been reported: intralesional injection of steroids, 5-fluorouracil (FU), or bleomycin; pressure garments; cryosurgery; laser therapy; and radiotherapy.3
Recently, Chen, et al.45 reported that heat shock protein 47 (Hsp47) is overexpressed in keloid fibroblasts, and that the overexpression of Hsp47 could induce excessive collagen accumulation by enhancing the synthesis and secretion of collagen.6 We previously confirmed that Hsp70 is overexpressed in keloid fibroblasts relative to normal fibroblasts.7 Also, other studies have reported that Hsp70 is overexperessed in keloid tissues.89 However, the functional role of Hsp70 in keloids has not been investigated yet. The Hsp70-family proteins act as chaperone molecules and protect cells from environmental stressors. They are stress-response proteins that are expressed under variety of stimuli, binding polypeptides to facilitate the correct folding, transport, and localization of the mature proteins.10 They also associate with denatured or partially unfolded proteins, helping the distressed proteins to refold. Thus, we hypothesize that Hsp70 plays a protective role in keloid fibroblasts, helping the cells to sustain a highly active metabolic state, allowing the cells to produce excessive collagen fibers and other extracellular matrix proteins, thus promoting pathogenic keloid development.
We obtained and cultured keloid-fibroblast samples from five patients in Severance Hospital, Yonsei University College of Medicine, Seoul, Korea as described previously.11 All patients provided informed written consent for the use of their tissues for research purposes, and the Institutional Review Board of Yonsei University Medical Center approved the tissue-harvesting procedure. None of patients had undergone previous keloid treatment.
Primary fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Grand Island, NY, USA) supplemented with heat-inactivated 10% fetal bovine serum (FBS), penicillin, and streptomycin. We used only cells before passage 5 in the experiments described here.
Immunohistochemistry (IHC) was performed in 10% formalin-fixed, paraffin-embedded keloid tissues. Tissues were pretreated with 3% hydrogen peroxide solution for 10 min, washed again with distilled water (DW) several times for 10 min each and incubated with 1xTBST for 5 min. Tissues were then treated with normal goat serum (Vector Laboratories, Burlingame, CA, USA) at room temperature (RT) for 1 h to prevent nonspecific reactions and incubated overnight with mouse anti-Hsp70 (ab6535; Abcam, Cambridge, UK) antibody and anti-transforming growth factor-beta (TGF-ß) (GTX110630; Gene Tex, San Antonio, TX, USA) diluted to 1:100. The tissues were then washed with 1xTBST and incubated for 30 min at RT with biotinylated secondary antibody solution from the Dako REAL™EnVision™Detection System (Dako, Copenhagen, Denmark), washed with DW, counter stained with hematoxylin (Sigma-Aldrigh, St. Louis, MO, USA), dehydrated and clarified by a conventional method, and prepared for examination under a light microscope.
We designed two Hsp70 siRNAs targeting the human Hsp70-1A sequence (Table 1) and synthesized them as double-stranded oligonucleotides (Bioneer, Daejeon, Korea). We seeded fibroblasts isolated from the tissue samples in 6-well plates at 70-80% confluency per well and cultured them for 24 h. We diluted the siRNAs in Opti-MEM (Gibco-BRL/Invitrogen, Carlsbad, CA, USA) to a 15 nM final concentration and then added 30 µL of Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) and incubated the mixture for 20 min at RT. The siRNA mixture was then added to the cells and the cells were incubated for 5 h in the incubator. At the end of the incubation period, the cell media were changed.
We extracted total RNA with an RNeasy plus mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. We synthesized cDNA using a Transcriptor first strand cDNA synthesis kit (Roche, Gipf-Oberfrick, Switzerland) and 1 µg of total RNA. We conducted real-time polymerase chain reaction (PCR) in triplicate using TaqMan master mix (Applied Biosystems, Foster City, CA, USA) and the Applied Biosystems 7500 Real-Time PCR System. We normalized the mRNA expression levels of Hsp70 (assay ID Hs00359163_s1), tissue inhibitor of metalloproteinase (TIMP)-1 (assay ID Hs00171558_m1), TIMP-2 (assay ID Hs00234278_m1), matrix metalloproteinase (MMP)-14 (assay ID Hs00 237119_m1), MMP-2 (assay ID Hs01548727_m1), collagen-I (assay ID Hs 00164004_m1) and collagen-III (assay ID Hs 00164103_m1) to that of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (assay ID Hs99999905_m1) housekeeping gene. We performed relative quantification using the 7500 software v2.0.1 (Applied Biosystems).
We collected keloid fibroblasts 24 h and 48 h after transfection to determine the total levels of Hsp70, MMP-2, MMP-14, TIMP-1, and TIMP-2. We separated proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred them to a nitrocellulose membrane, which was blocked 1 h with 5% skim milk in 1xTBST, and then incubated them overnight at 4℃ with monoclonal mouse anti-human Hsp70 antibody (1:1000, Abcam), MMP-2 antibody (1:1000, Abcam), MMP-14 antibody (1:1000, Abcam), TIMP-1 antibody (1:1000, Abcam), and TIMP-2 antibody (1:1000, Abcam). The primary antibody was detected with horseradish peroxidase-conjugated secondary antibody goat anti-mouse or rabbit (1:5000, Cell signaling, Beverly, MA, USA) and chemiluminescent luminal (Las 4000 mini; Fuji, Tokyo, Japan). Immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus; Amersham International plc, Little Chalfont, UK), followed by autoradiography (Hyperfilm EC; Amersham International plc). Finally, immunoblot signals were quantified using a computer-imaging program (GelScopeTM, Imageline Inc., Gardena, CA, USA).
The total collagen content of 100 µL of media from each cell culture after siRNA transfection was determined using the Sircol Collagen Assay Kit (Biocolor, Belfast, Northern Ireland) according to the manufacturer's instructions. Briefly, we added 1 mL of Sircol dye reagent to bind the collagen in each sample and then mixed the solutions for 30 min, and then centrifuged the solutions at 12000 rpm for 10 min, resuspended the pellets in 1 mL of alkali reagent and read the absorbance at 550 nm with a spectrophotometer. A standard collagen solution was used to construct a standard curve.
A tetrazolium-based colorimetric assay (MTT) assay was used to measure cell viability after different treatments. We added 20 µL of MTT solution (5 mg/mL, Sigma-Aldrich) to each well at each designated time point and then incubated the wells for 4 h. Then, 250 µL of dimethyl sulphoxide (DMSO) were added to dissolve the purple crystalline products of the MTT substrates, incubated the mixtures for 30 min, and measured absorbance at 570 nm using a plate reader.
Dense collagen fibers were noted in keloid tissue. Immunohistochemical staining of keloids and adjacent normal dermal tissue revealed that the expressions of TGF-ß and Hsp70 were markedly increased along with dense collagen fibers in keloid tissue. Increased expression of TGF-ß and Hsp70 was also noted under adjacent normal dermal tissue (Fig. 1).
There was no statistically significant difference in keloid fibroblasts viability after Hsp70 siRNA transfection (Fig. 2A). Neither the scrambled siRNA nor the Hsp70 siRNAs influenced the viability of the keloid fibroblasts, indicating that the suppression of Hsp70 had no influence on keloid fibroblast viability.
The quantitative real-time PCR and western blot results showed that siRNAs effectively suppressed both Hsp70 mRNA and protein levels (p<0.05) (Fig. 2B and C). Hsp70 siRNA-1 suppressed Hsp70 mRNA levels more consistently than Hsp70 siRNA-2. Therefore, we selected Hsp70 siRNA-1 for further investigation.
Fig. 3 shows that both intracellular collagen production and extracellular collagen secretion were markedly suppressed after 24 h and 48 h of transfection. Relative to those in the control cells transfected with a scrambled siRNA, the collagen-I and collagen-III mRNA levels were reduced by 76% and 71%, respectively, 24 h after transfection (p<0.05) and by an additional 79% and 81%, respectively, 48 h after transfection (p<0.05) (Fig. 3A). The collagens secretion was suppressed in the cultures transfected with Hsp70 siRNA-1, relative to the control cultures, 48 h after transfection (p<0.05), but not 24 h after transfection (Fig. 3B).
In the present study, we found that transfection of keloid fibroblasts with Hsp70 siRNA knocked down Hsp70 expression, resulting in reduced expression of collagen-I, collagen-III, MMP-14, TIMP-1, and TIMP-2, and that the siRNA-mediated Hsp70 knockdowns did not affect the viability of the keloid fibroblasts, suggesting a possible role for Hsp70 in keloid development.
Heat shock proteins are stress-response proteins that are expressed under a variety of stimuli and are binding polypeptides to facilitate the correct folding, transport, and localization of the mature proteins.12 They also associate with denatured or partially unfolded proteins, helping the distressed proteins to refold. Among the many heat shock proteins, the members of the Hsp70 family are the most prominent and best characterized, and are well known to have cytoprotective,131415 anti-inflammatory,16 and molecular chaperone activities.10 Previously, increased expression of Hsp70 in keloid tissues has been reported in other studies. Totan, et al.8 showed overexpression of Hsp27, Hsp47, and Hsp70 in keloid tissues, and Javad and Day9 showed up-regulated Hsp60 and Hsp70 in keloid tissue using a protein profiling. In support of these reports, we previously showed that Hsp70 is also increased in cultured fibroblasts.7 However, the functional role of Hsp70 in collagen has not yet been investigated.
The overproduction of extracellular matrix proteins and especially that of collagen is a prominent phenomenon in keloids. Previous studies suggest that the ratio of collagen-I to collagen-III is altered in keloids.1718 The levels of collagen-I generally increase in keloid fibroblasts;1819 whereas the levels of collagen-III vary: Peltonen, et al.20 and Ala-Kokko, et al.19 found unaltered collagen-III mRNA levels in keloids, while Syed, et al.21 and Naitoh, et al.17 found increased collagen-III mRNA and protein levels in keloids. Those authors suggested that the variation in collagen-III mRNA levels among different studies may be explained by the heterogeneity within the keloid lesion itself.
In our study, we focused on the mechanism of excessive collagen deposition that leads to keloid formation. Previously, we reported that the level of Hsp70 protein is increased in keloid fibroblasts compared with that in normal fibroblasts,7 leading us to examine the role of Hsp70 in keloid pathogenesis in the present study, especially the excessive deposition of collagen, by suppressing Hsp70 expression in keloid fibroblasts.
We used siRNAs to suppress Hsp70 expression. As shown in previous studies,22 RNA interference using siRNA is a useful biological strategy to study keloid pathogenesis. Therefore, we designed two siRNAs and transfected them into keloid fibroblasts, thereby successfully suppressing the expression of Hsp70. Of the two siRNAs, Hsp70 siRNA-1 more constantly suppressed the expression of Hsp70 in repeated experiments. Therefore, we decided to further evaluate Hsp70 knockdown using only siRNA-1. After successfully down-regulating Hsp70 expression, we detected the collagen production to reflect the condition of collagen accumulation in keloids. We found that the mRNA levels of collagen-I and collagen-III decreased significantly, corresponding to the down-regulation of Hsp70. We also found that the amount of extracellular collagen in the culture media was significantly decreased. Taken together, our results suggest that the suppression of Hsp70 by siRNAs could inhibit the synthesis and secretion of collagen, suppressing excessive collagen accumulation found in keloids.
When Hsp70 is overexpressed in animal models by genetic or pharmacologic induction, it is protective of various diseases, such as gastric and small-intestinal lesions, inflammatory bowel diseases, ultraviolet-induced skin damage, and Alzheimer's disease.23242526272829 Recently, Tanaka, et al.30 and Namba, et al.31 demonstrated that Hsp70 has a protective role in pulmonary fibrosis in a genetically altered mouse model. In contrast to previous studies, our data showed increased expression of Hsp70 in keloids and the possible regulatory role in collagen synthesis. The contrasting results could be due to differences in the cell types: human lung adenocarcinoma cell lines (A539, H1975, and PC9)30 and genetically altered mouse cells31 versus human keloid fibroblasts. The overexpression of Hsp70 in epithelial cells may play a protective role via cytoprotective and anti-inflammatory effects; however, the overexpression of Hsp70 in altered fibroblasts, such as keloid fibroblasts, may lead to the pathogenic maintenance of excessive extracellular matrix-protein production.
Another heat shock protein, Hsp47 is a collagen-specific molecular chaperone that plays a critical role in normal procollagen biosynthesis in mammals. Chen, et al.6,32 demonstrated that the overexpression of Hsp47 in keloid fibroblasts may induce excessive collagen accumulation by enhancing the synthesis and secretion of collagen. Their results are in good agreement with our present results: overexpressed heat shock proteins in keloid fibroblasts might cause excessive collagen accumulation, leading to keloid formation.
Collagenases such as MMPs and TIMPs are involved in creating an imbalance between the production and the degradation of collagen in keloids. Several MMPs have been shown to mediate the breakdown of collagen-I and III.33 MMP-2 and MMP-9 activities persist after wound closure and seem to play a critical role in the remodeling phase.34 Keloids were previously found to contain high levels of MMP-2 and low or normal levels of MMP-9.3536 MMP-2, secreted as latent pro-MMP-2, requires activation in the presence of TIMP-2 and surface MMP-14 binding.37 This TIMP-2:MMP-14 complex acts as a receptor for pro-MMP-2 at the cell surface and converts pro-MMP-2 to active MMP-2.38 Previous studies indicate that the activation of MMP-2 via the increased expression of MMP-14 and TIMP-2 is related to tumor progression and invasion.39404142434445 It has also been demonstrated that increased MMP-2 activity in keloids coupled with TIMP-2 and MMP-14 expression by keloid fibroblasts can contribute to the invasion of surrounding tissues by keloid fibroblasts.36 We found that the down-regulation of Hsp70 increased levels of MMP-2 mRNAs and decreased levels of TIMP-1, TIMP-2, and MMP-14 mRNAs and proteins. Although the MMP-2 mRNA levels increased, the decreased levels of MMP-14 and TIMP-2 will likely suppress the conversion of pro-MMP-2 to active MMP-2, thereby reducing the ECM degradation and invasion of the keloid fibroblasts into peripheral normal region.
In summary our results, together with our previous results, support the hypothesis that the overexpression of Hsp70 in keloid fibroblasts might play a regulatory role in the excessive collagen production and secretion in keloids. Hence, the down-regulation of Hsp70 could be a therapeutic option for keloids, suppressing collagen deposition and preventing keloid progression.
Figures and Tables
Fig. 1
Immunohistochemical staining of keloids and adjacent normal dermal tissues. Abnormal dense collagen fibers were extending over clinical keloid margin (dotted line). The expression of Hsp70 and TGF-β1 were also increased along with extending collagen fibers (×100, scale bar: 1 mm). Each experiment was performed in triplicate; representative data are shown here. Hsp70, heat shock protein 70; TGF-β1, transforming growth factor-beta1.
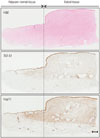
Fig. 2
The expression of Hsp70 was down-regulated by Hsp70 siRNA transfection. (A) MTT viability of keloid fibroblasts after siRNA transfection. Results are shown as mean±SD. Experiment was performed in triplicate. (B) Hsp70 mRNA levels determined by RT-PCR; results are shown as mean±SD. Experiment was performed in two sets of triplicate. (C) Detection of the Hsp70 protein in keloid fibroblasts by western blot analysis 24 h and 48 h after transfection (*p<0.05). Each experiment was performed in triplicate; representative data are shown here. Hsp70, heat shock protein 70; siRNA, small interfering RNA; MTT, tetrazolium-based colorimetric assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, real-time polymerase chain reaction.
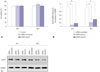
Fig. 3
Collagen mRNA and protein levels in keloid fibroblasts decreased significantly, corresponding to the down-regulation of Hsp70. (A) Expression of collagen-I and III mRNAs after Hsp70 siRNA transfection. Each experiment was performed in two sets of triplicate. (B) Histogram of the extracellular secreted-collagen content of keloid fibroblast cultures by Sircol collagen assay 24 h and 48 h after transfection; results are shown as mean±SD (*p<0.05). Each experiment was performed in triplicate. Hsp70, heat shock protein 70; siRNA, small interfering RNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.

Fig. 4
Matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) 24 h and 48 h after transfection. (A) Expression of MMP and TIMP mRNAs after Hsp70 siRNA transfection: MMP-14, TIMP-1, and TIMP-2 mRNAs were significantly suppressed; however, MMP-2 was not; results are shown as mean±SD. Each experiment was performed in two sets of triplicate. (B) Western-blot analysis of MMP-2, MMP-14, TIMP-1, and TIMP-2 protein levels (*p<0.05). Each experiment was performed in triplicate. Hsp70, heat shock protein 70; siRNA, small interfering RNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2010-0010475). This study was also supported by a faculty research grant of Yonsei University College of Medicine (6-2010-0057).
References
1. Mukhopadhyay A, Tan EK, Khoo YT, Chan SY, Lim IJ, Phan TT. Conditioned medium from keloid keratinocyte/keloid fibroblast coculture induces contraction of fibroblast-populated collagen lattices. Br J Dermatol. 2005; 152:639–645.
2. Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998; 4:19–24.

3. Poochareon VN, Berman B. New therapies for the management of keloids. J Craniofac Surg. 2003; 14:654–657.

4. Chen W, Fu X, Sun X, Sun T, Zhao Z, Sheng Z. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003; 113:208–216.

5. Chen W, Fu XB, Ge SL, Sun XQ, Zhou G, Zhao ZL, et al. Development of gene microarray in screening differently expressed genes in keloid and normal-control skin. Chin Med J (Engl). 2004; 117:877–881.
6. Chen JJ, Zhao S, Cen Y, Liu XX, Yu R, Wu DM. Effect of heat shock protein 47 on collagen accumulation in keloid fibroblast cells. Br J Dermatol. 2007; 156:1188–1195.

7. Lee JH, Shin JU, Jung I, Lee H, Rah DK, Jung JY, et al. Proteomic profiling reveals upregulated protein expression of hsp70 in keloids. Biomed Res Int. 2013; 2013:621538.

8. Totan S, Echo A, Yuksel E. Heat shock proteins modulate keloid formation. Eplasty. 2011; 11:e21.
9. Javad F, Day PJ. Protein profiling of keloidal scar tissue. Arch Dermatol Res. 2012; 304:533–540.

10. Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996; 1:23–28.

11. Zhang GY, Yi CG, Li X, Ma B, Li ZJ, Chen XL, et al. Troglitazone suppresses transforming growth factor-beta1-induced collagen type I expression in keloid fibroblasts. Br J Dermatol. 2009; 160:762–770.

13. Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996; 111:345–357.

14. Mathew A, Morimoto RI. Role of the heat-shock response in the life and death of proteins. Ann N Y Acad Sci. 1998; 851:99–111.

15. Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010; 40:253–266.

16. Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007; 179:1236–1244.

17. Naitoh M, Hosokawa N, Kubota H, Tanaka T, Shirane H, Sawada M, et al. Upregulation of HSP47 and collagen type III in the dermal fibrotic disease, keloid. Biochem Biophys Res Commun. 2001; 280:1316–1322.

18. Uitto J, Perejda AJ, Abergel RP, Chu ML, Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985; 82:5935–5939.

19. Ala-Kokko L, Rintala A, Savolainen ER. Collagen gene expression in keloids: analysis of collagen metabolism and type I, III, IV, and V procollagen mRNAs in keloid tissue and keloid fibroblast cultures. J Invest Dermatol. 1987; 89:238–244.

20. Peltonen J, Hsiao LL, Jaakkola S, Sollberg S, Aumailley M, Timpl R, et al. Activation of collagen gene expression in keloids: co-localization of type I and VI collagen and transforming growth factor-beta 1 mRNA. J Invest Dermatol. 1991; 97:240–248.

21. Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011; 164:83–96.

22. Gao Z, Wang Z, Shi Y, Lin Z, Jiang H, Hou T, et al. Modulation of collagen synthesis in keloid fibroblasts by silencing Smad2 with siRNA. Plast Reconstr Surg. 2006; 118:1328–1337.

23. Asano T, Tanaka K, Yamakawa N, Adachi H, Sobue G, Goto H, et al. HSP70 confers protection against indomethacin-induced lesions of the small intestine. J Pharmacol Exp Ther. 2009; 330:458–467.

24. Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, et al. Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011; 31:5225–5234.

25. Matsuda M, Hoshino T, Yamashita Y, Tanaka K, Maji D, Sato K, et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J Biol Chem. 2010; 285:5848–5858.

26. Suemasu S, Tanaka K, Namba T, Ishihara T, Katsu T, Fujimoto M, et al. A role for HSP70 in protecting against indomethacin-induced gastric lesions. J Biol Chem. 2009; 284:19705–19715.

27. Tanaka K, Mizushima T. Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperthermia. 2009; 25:668–676.

28. Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, et al. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007; 282:23240–23252.

29. Tanaka K, Tsutsumi S, Arai Y, Hoshino T, Suzuki K, Takaki E, et al. Genetic evidence for a protective role of heat shock factor 1 against irritant-induced gastric lesions. Mol Pharmacol. 2007; 71:985–993.

30. Tanaka K, Tanaka Y, Namba T, Azuma A, Mizushima T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2010; 80:920–931.

31. Namba T, Tanaka K, Hoshino T, Azuma A, Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PLoS One. 2011; 6:e27296.

32. Chen JJ, Jin PS, Zhao S, Cen Y, Liu Y, Xu XW, et al. Effect of heat shock protein 47 on collagen synthesis of keloid in vivo. ANZ J Surg. 2011; 81:425–430.

33. Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007; 15:Suppl 1. S46–S53.

34. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005; 153:295–300.

35. Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen. 1999; 7:166–171.

36. Imaizumi R, Akasaka Y, Inomata N, Okada E, Ito K, Ishikawa Y, et al. Promoted activation of matrix metalloproteinase (MMP)-2 in keloid fibroblasts and increased expression of MMP-2 in collagen bundle regions: implications for mechanisms of keloid progression. Histopathology. 2009; 54:722–730.

37. Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, et al. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J Biol Chem. 1998; 273:1216–1222.

38. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998; 273:871–880.

39. Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006; 6:1215–1225.

40. Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000; 87:12–19.

41. Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000; 191:245–256.

42. Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci U S A. 1997; 94:7959–7964.

43. Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995; 55:3263–3266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download