Abstract
Purpose
To evaluate a recently marketed commercial glycoprotein enzyme-linked immunosorbent assay (gpEIA) kit, the VaccZyme™ VZV gpEIA, for measuring the immunity of varicella-vaccinated children.
Materials and Methods
We investigated the accuracy and reproducibility of the VaccZyme™ VZV gpEIA kit for the detection of antibodies to VZV. We also examined the sensitivity, specificity, and correlation between antibody titers calculated with gpEIA versus fluorescent antibody to membrane antigen (FAMA) by using sera of 349 children, ranging from 1 to 6 years old.
Results
VaccZyme™ VZV gpEIA gave precise and reproducible intra- and inter-assay results. FAMA and gpEIA titers showed a linear correlation (Pearson correlation coefficient=0.987). The sensitivity and specificity of the VaccZyme™ gpEIA was 31.4% and 100%, respectively, when the guidelines of the gpEIA (<100 mIU/mL) and FAMA 1:4 were adopted as cutoff values. However, the maximum sensitivity and specificity were 88.9% and 95.1%, respectively, with the highest correlation (κ=0.840), if the cutoff values were set with gpEIA at 49.7 mIU/mL and FAMA 1:16.
Conclusion
These results demonstrate that the VaccZyme™ VZV gpEIA kit gave precise and reproducible data for measuring antibody titer after varicella vaccination. The results also showed that the antibody titer calculated with the VaccZyme™ gpEIA kit strongly correlated with the FAMA titer. However, cutoff values should be re-optimized for the evaluation of vaccine immunity.
The varicella vaccine has been selectively used in Korea since 1988 and has been included in a routine vaccination program for children (with a single dose schedule) since 2005. However, breakthrough infections have occurred and it is necessary to evaluate children for their immune status against varicella. Since the development of a varicella vaccine in the 1970s, various methods used to assess vaccine efficacy include the complement-fixation test, neutralization test, fluorescent antibody to membrane antigen (FAMA) test, immune adherence hemagglutination test, enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence assay, radioimmunoassay, glycoprotein enzyme-linked immunosorbent assay (gpEIA), latex agglutination, and time-resolved fluorescence immunoassay.1-9 However, the evaluation of immunogenicity for the varicella vaccine is hindered by several challenges. For example, the protective antibody detection methods for varicella-zoster virus (VZV) do not satisfy all the conditions essential for vaccine evaluation. These conditions include objectivity, sensitivity, specificity, precision, reproducibility, ruggedness, high-throughputness, automation, and commercial availability.10
Moreover, the attenuated varicella vaccine usually induces a lower level of VZV-specific antibody titer than a natural infection.11-13 This diminished response necessitates a very sensitive measurement method. Furthermore, the measurement of protective immunity against varicella is very important in the evaluation of vaccine efficacy.14 Even though the FAMA test is regarded as a gold standard for measuring a protective antibody response against varicella, this test cannot be used as a routine assay because it is very labor-intensive and not amenable to automation.3,10,15-17 Various methods have been developed to overcome this hurdle. One of the most reliable methods is a glycoprotein ELISA (gpELISA) that detects and quantifies antibodies against VZV glycoproteins.18,19 The evaluation of varicella vaccine immunity has been done in the United States using a gpELISA developed by Merck, Sharp, & Dohme Research Laboratories.20-23 However, this kit is not commercially available.
Furthermore, the interpretation guidelines for protective antibody titer levels after vaccination have not been well standardized between methods or companies. Indeed, different units and cutoff values can exist within a method, depending on the researchers or manufacturers involved. Thus, it is very difficult to compare study results. Generally, a FAMA VZV antibody titer ≥1:4 or a Merck gpELISA ≥5 EU/mL are thought to correlate with protection against varicella after vaccination.24,25 However, these antibody titers, six weeks after vaccination, may not guarantee life-long protection because the antibody titer induced by the vaccine wanes with time.26-29
Recently, the VaccZyme™ VZV gpEIA kit has been launched and this kit uses the VZV WHO international standard as a calibrator. Therefore, we investigated this kit for the evaluation of immunity in varicella-vaccinated children.
To evaluate the commercial VZV gpEIA kit, a total 349 sera were included. This work was approved by the Institutional Review Board at Yeungnam University Medical Center. Parental consent was obtained prior to blood collection based on the protocol PCR-09-23 and protocol PCR 11-199. The positive panel included sera from 305 varicella vaccinees who had been enrolled in 14 day-care centers at Gyeongsan City. They were 2-6 years old (mean 3.7 years). They were recruited during annual health examination from April 2009 to June 2009. The negative panel was obtained from 44 residual sera which were used to confirm hepatitis B virus (HBV) seroconversion after HBV vaccination. Children had neither a history of varicella disease nor a varicella vaccination and they were 5-12 months old. They were enrolled from January 2012 to December 2012 at Yeungnam University Hospital. All blood samples were collected and centrifuged for 20 min at 2000 rpm. After centrifugation, sera were extracted and stored at -70℃.
Antibody titer was measured using the VaccZyme™ VZV gpEIA kit (Binding Site, Birmingham, UK), which measures IgG antibodies specific to viral envelope glycoproteins. There are two kinds of VaccZyme™ VZV gpEIA kits with different detection ranges: Low (10-810 mIU/mL) and Screening (0.5-10 IU/mL) kits. All samples were tested using the Low kit first. Samples beyond the detection limit of the Low kit (>810 mIU/mL) were re-analyzed using the Screening kit. Procedures were followed according to the manufacturer's instructions. According to the interpretation guidelines of the kit, an antibody titer <100 mIU/mL, 100-150 mIU/mL, and ≥150 mIU/mL indicated susceptible to infection, equivocal, and protective level, respectively. In this study, both equivocal and protective levels (≥100 mIU/mL) were considered as positive to calculate sensitivity and specificity against the FAMA test.
The gpEIA and FAMA tests were performed (separately and independently) with the expertise of different personnel blind to results obtained from the other assay. If discrepancies occurred between the FAMA test and the gpEIA results, these two tests were repeated.
The intra-assay was performed in ten replicates using ten sera within the range of the calibration curve in a single run. The inter-assay reproducibility between nine or ten runs was evaluated with duplicate samples; assays were run on a separate day. Two controls (high and low), five calibrators (10, 30, 90, 270, 810 mIU/mL), and sera from ten children were included in each run.
The MGLu human embryonic lung fibroblast cell line was provided from the Mogam Biotechnology Institute (Yongin, Korea). MGLu cells were cultured and infected with Varilrix™ (GSK, UK) as described previously.30 When 60-70% of the cells showed a cytopathic effect, cells were harvested and used as a FAMA antigen.30 The FAMA test was performed according to William's method with some modification.3,30 WHO international standard for VZV immunoglobulin (NIBSC W1044, UK) was used as a positive reference serum. Phosphate buffered saline and sera (gpEIA titer <30 mIU/mL) from two children, who had neither been vaccinated nor experienced varicella, were used as negative controls. Alexa Fluor®488 goat anti-human IgG (Molecular Probes, New York, NY, USA) was used as a secondary antibody. Cells were observed using an Axioscope fluorescent microscope (Carl Zeiss, Germany). Depending on the fluorescent intensity of the ring structure around the cell surface, the cells were graded as - (negative), W (weak), 1+, 2+, and 3+. Negative cells had no fluorescence or non-specific fluorescence. Weak showed specific-looking but very weak fluorescence with partial ring, 1+ showed specific clear fluorescence with complete thin ring structure, 2+ showed bright and specific fluorescence with complete ring structure, and 3+ showed brilliant fluorescence with complete thick ring structure.
ANOVA was used to evaluate the precision and reproducibility of intra- and inter assays. Pearson's correlation coefficient was calculated to confirm a linear relationship between gpEIA and FAMA titers using IBM SPSS Statistics 19.0 (IBM corporation, New York, NY, USA). A receiver operator characteristic (ROC) curve created in the MedCalc program (MedCalc Software, Belgium) was used to determine the most appropriate cutoff values of the gpEIA and FAMA test. Accuracy was determined by the area under the ROC curve (AUC) and considered significant at a minimum value of 0.5. The inter-rater agreement statistic (Kappa value) was calculated by the MadCalc program.
Although the varicella vaccine has been used for over 20 years, a lack of commercially available methods to evaluate protective immunity has hampered the determination of mass immunogenicity after vaccination. Recently, a commercially developed and marketed gpEIA kit, the VaccZyme™ VZV gpEIA, has become available. VaccZyme™ VZV gpEIA Low and Screening kits used in this experiment are coated with antigens formulated with purified VZV glycoproteins. The Screening kit is intended for diagnostic purposes; therefore, it has a high detection range (0.5-10 IU/mL). On the other hand, the Low kit is used to evaluate vaccine immunogenicity; therefore, it has a low range (10-810 mIU/mL). The kits use WHO international standard VZV immunoglobulin as a calibrator; therefore, it is possible to compare the VaccZyme™ gpEIA antibody titer with different assay methods if researchers use this standard immunoglobulin as a reference serum. We examined the suitability of the Low kit for the evaluation of immunity in vaccinated children. First, we evaluated the precision and reproducibility of the kit by an intra-assay and an inter-assay.
The coefficients of variation (CVs) of ten sera for the intra-assay assessment were between 2.0-18.1%, and all samples, except one, were within the permitted range (≤15%) of manufacturer's instructions (Table 1). An ANOVA was used to compare the mean values of ten replicate test results (using ten sera), and the p-value was determined to be 1.000. Therefore, the error within one plate can be deemed negligibly small. In the inter-assay analysis, the CVs of the five calibrators and two controls were all <15% (range, 5.63-11.22%) (Table 2). However, the CVs of three, among ten sample sera, exceeded 15% (range, 6.2-23.3%) (Table 3). An ANOVA conducted in the same fashion as the intra-assay assessment yielded a p-value of 1.000; no significant differences among repeats were evident. Therefore, the precision and reproducibility of the VaccZyme™ VZV gpEIA kit were acceptable.
The original FAMA test, developed by Williams, et al.,3 uses unfixed cells to prepare the antigen. However, unfixed cells cannot be stored for a long time, therefore, some laboratories used glutaraldehyde for cell fixing.31-33 Besides fixation methods, cell lines, VZV strains, reference sera, and cutoff values are different between laboratories.3,20,24,30,31,33-35 Williams, et al.3 used a wild type VZV Ellen strain, and recently the Oka vaccine strain has been used in some laboratories.3,30,33,35 In this study, we used unfixed human embryonic lung fibroblasts infected with the VZV Oka vaccine strain for FAMA antigen and WHO international standard VZV immunoglobulin as a positive reference serum.
Depending on the fluorescent intensity of the ring structure around the cell surface, cells were graded as - (negative), W (weak), 1+, 2+, and 3+. Fluorescent intensities of 1000 to 50 mIU/mL of reference serum were almost identical, therefore, ≥50 mIU/mL was graded as "3+". The fluorescent intensity of 49 to 10 mIU/mL was "2+", 9 to 5 mIU/mL were "1+", 4 to 2 mIU/mL were "weak", and 1 mIU/mL was "negative" (Fig. 1A-F). Therefore, cells graded ≥"1+" were regarded as FAMA-positive and corresponded to ≥5 mIU/mL. This result is comparable to a previous report.35 Confocal microscopy revealed a bright fluorescent ring on the cell membrane in a single optical section (Fig. 1G), and punctated fluorescence was evenly distributed over the whole surface of VZV-infected cells in a z-stack (Fig. 1H).
The interpretation guidelines of VaccZyme™ VZV gpEIA suggest that an antibody titer <100 mIU/mL is susceptible to varicella. If a gpEIA <100 mIU/mL and FAMA 1:4 were applied as cutoff values, the sensitivity and specificity of gpEIA were 31.4% and 100%, respectively (Table 4). Generally, FAMA-positive at a 1:4 serum dilution was considered a protective level for varicella infection. Therefore, an antibody titer ≥20 mIU/mL should be theoretically protective. Such a low titer reflects the high sensitivity of the FAMA test. However, the interpretation guidelines of VaccZyme™ suggest that 100-150 mIU/mL is equivocal and ≥150 mIU/mL is protective in both Screening and Low kits. Therefore, the antibody titers indicating protective levels show great discrepancies between the FAMA and VaccZyme™ gpEIA. This is probably due to VaccZyme™ gpEIA guidelines derived from the data of adults naturally infected by VZV in the United Kingdom. It is generally known that the varicella vaccine induces a lower immunogenicity than natural infection. Thus, it is possible that the VaccZyme™ kit interpretation guidelines may be unsuitable for the evaluation of vaccinated children. Indeed, the Merck gpELISA kit, developed for the evaluation of the VZV vaccine, suggests that >5 EU/mL (equivalent to 10 mIU/mL) indicates a protective level.36 Therefore, the interpretation guidelines of the Low kit should be made using data from vaccinated children.
Because there was a great difference between the cutoff values of the FAMA and VaccZyme™ VZV gpEIA, we evaluated the relationship of antibody titers from 349 children obtained by the two methods. The Pearson's correlation coefficient was 0.987, showing a very good linear correlation (Fig. 2).
Recently, the immunogenicity of the VZV vaccine was evaluated by the FAMA test in two groups, using unfixed cells, with a 1:4 cutoff value.24,35 The seroconversion rate of the vaccinee, measured by FAMA, was 76% which was much lower than previous seroconversion rates measured by the Merck gpELISA.24,25,37,38 Michalik, et al. suggested that the low endpoint of Merck gpELISA might cause false-positives and an overestimation of the seroconversion rate.24 On the other hand, Kim, et al.35 reported that the seroprevalence rate of vaccinated children measured by FAMA was 83.6%, and the seropositive rate of those children was 44.8% when measured by the conventional Enzygnost® (Siemens, Marburg, Germany) ELISA. It is well known that commercial whole cell ELISAs, such as the Enzygnost® ELISA, are less sensitive than the FAMA test for the detection of antibodies after vaccination. Therefore, conventional ELISAs might underestimate vaccine seroconversion rates.
In this study, if a FAMA 1:4 and gpEIA <100 mIU/mL were set as cutoff values, 58.9% of susceptible levels in gpEIA samples were FAMA positive (Table 4). However, the linear correlation between gpEIA and FAMA titers was very high (r=0.987), suggesting that either the cutoff value of gpEIA is too high or the cutoff value of FAMA is low.
2006 European Sero-Epidemiology Network 2 (ESEN2) guideline for the diagnosis of varicella using conventional ELISA suggested <50 mIU/mL is negative.39 Recently developed gpEIA kits, Viron\Serion and RIDASCREEN, use the same cutoff values as the 2006 ESEN2 guidelines for both natural infection and post vaccination.13,18 Even though a FAMA ≥1:4 is considered a positive antibody response for varicella,40 long-term follow-up studies of varicella vaccinees have shown that a FAMA titer over 1:8 or 1:16 can be protective antibody levels for varicella.41,42 Nevertheless, some people with FAMA ≥1:8 experienced varicella infection.26,29
We reanalyzed our data using different cutoff values. If gpEIA <100 mIU/mL was applied as a cutoff value, the sensitivity and specificity of gpEIA were 39.8% and 100% at FAMA 1:8, and 48.6% and 98% at FAMA 1:16, respectively (Table 4). If gpEIA <50 mIU/mL was adopted as a cutoff value, the sensitivity and specificity of gpEIA were 57.6% and 98.2% at FAMA 1:4, 73.1% and 98.8% at FAMA 1:8, and 88.9% and 95.1% at FAMA 1:16, respectively (Table 4). Maximum sensitivity and specificity were calculated with the combination of different cutoff values for gpEIA and FAMA by the MedCalc program. Maximum sensitivity and specificity were 77.9% and 92.0% at FAMA 1:4 and gpEIA titer of 34.3 mIU/mL, 87.6% and 93.9% at FAMA 1:8 and gpEIA 39.0 mIU/mL, and 88.9% and 95.1% at FAMA 1:16 and gpEIA 49.7 mIU/mL (Table 5). The AUC using the ROC curve was highest (0.96) at FAMA 1:8 and gpEIA 39.0 mIU/mL, and the Kappa value was highest (0.84) at FAMA 1:16 and gpEIA 49.7 mIU/mL (Table 5).
The protective level of gpELISA antibody to varicella was initially set at 0.625 EU/mL by Merck, Sharp & Dohme Research Laboratories at the beginning of VZV vaccine clinical trials in the United States.20,21 However, based on accumulating data, the level was changed to 5 EU/mL.23 Nevertheless, several breakthrough infections have been reported in children with ≥5 EU/mL six weeks after vaccination.25,27,43-45 In this study, the most appropriate cutoff value for the VaccZyme™ VZV gpEIA seems to be 50 mIU/mL. However, the cutoff value for the protective level of the varicella vaccine should be considered together with long-term follow-up epidemiological data.
In conclusion, this study examined the commercial VaccZyme™ gpEIA Low kit for the evaluation of immunity after varicella vaccination. The precision and reproducibility of the VaccZyme™ VZV gpEIA kit are acceptable, and there is a very high correlation between gpEIA and FAMA titers. However, the interpretation guidelines of the VaccZyme™ gpEIA Low kit should be re-optimized for the evaluation of post-vaccination immunity.
Figures and Tables
Fig. 1
Microphotograph images of VZV infected MGLu cell in FAMA test. The images were taken by Axioscope fluorescent microscope (A-F) and Leica SP2 confocal microscope (G and H). Reading criteria for FAMA using WHO standard VZV IgG was set as follows 1 IU/mL as "3+" (A); 500 mIU/mL as "3+" (B); 31.2 mIU/mL as "2+" (C); 7.8 mIU/mL as "1+" (D); 3.9 mIU/mL as "weak" (E); 0.98 mIU/mL as "negative" (F); single plane (G); z-stack (H). VZV, varicella-zoster virus; FAMA, fluorescent antibody to membrane antigen; MGLu, human embryonic lung fibroblast.
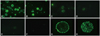
Fig. 2
Correlation between gpEIA and FAMA titers of 349 children without VZV histories (Pearson correlation coefficient=0.987). gpEIA, glycoprotein enzyme-linked immunosorbent assay; FAMA, fluorescent antibody to membrane antigen; VZV, varicella-zoster virus.

ACKNOWLEDGEMENTS
The authors would like to thank Kyung Min Lee and Jin Hee Choi for technical supports. This research was supported by grant 09122KFDA423 from the Korea Food & Drug Administration in 2009.
References
1. Schmidt NJ, Lennette EH, Shon CW, Shinomoto TT. A complement-fixing antigen for varicella-zoster derived from infected cultures of human fetal diploid cells. Proc Soc Exp Biol Med. 1964; 116:144–149.

2. Caunt AE, Shaw DG. Neutralization tests with varicella-zoster virus. J Hyg (Lond). 1969; 67:343–352.

3. Williams V, Gershon A, Brunell PA. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974; 130:669–672.

4. Gershon AA, Kalter ZG, Steinberg S, Kuhns WJ. Detection of antibody to Varicella-Zoster virus by immune adherence hemagglutination. Proc Soc Exp Biol Med. 1976; 151:762–765.

5. Forghani B, Schmidt NJ, Dennis J. Antibody assays for varicella-zoster virus: comparison of enzyme immunoassay with neutralization, immune adherence hemagglutination, and complement fixation. J Clin Microbiol. 1978; 8:545–552.

6. Arvin AM, Koropchak CM. Immunoglobulins M and G to varicella-zoster virus measured by solid-phase radioimmunoassay: antibody responses to varicella and herpes zoster infections. J Clin Microbiol. 1980; 12:367–374.

7. Keller PM, Lonergan K, Neff BJ, Morton DA, Ellis RW. Purification of individual varicella-zoster virus (VZV) glycoproteins gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J Virol Methods. 1986; 14:177–188.

8. Steinberg SP, Gershon AA. Measurement of antibodies to varicella-zoster virus by using a latex agglutination test. J Clin Microbiol. 1991; 29:1527–1529.

9. Maple PA, Gray J, Breuer J, Kafatos G, Parker S, Brown D. Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial enzyme immunoassays and Merck glycoprotein enzyme immunoassay. Clin Vaccine Immunol. 2006; 13:214–218.

10. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996; 10:507–527.

11. LaRussa PS, Gershon AA, Steinberg SP, Chartrand SA. Antibodies to varicella-zoster virus glycoproteins I, II, and III in leukemic and healthy children. J Infect Dis. 1990; 162:627–633.

12. Ndumbe PM, Cradock-Watson J, Levinsky RJ. Natural and artificial immunity to varicella zoster virus. J Med Virol. 1988; 25:171–178.

13. Jenke AC, Klein S, Baiker A, Wirth S, Sander M, Noelting C, et al. Serologic analysis of the IgG antibody response in children with varicella zoster virus wild-type infection and vaccination. Pediatr Infect Dis J. 2012; 31:1148–1152.

14. Kreth HW, Lee BW, Kosuwon P, Salazar J, Gloriani-Barzaga N, Bock HL, et al. Sixteen years of global experience with the first refrigerator-stable varicella vaccine (Varilrix). BioDrugs. 2008; 22:387–402.

15. Hambleton S, Gershon AA. Preventing varicella-zoster disease. Clin Microbiol Rev. 2005; 18:70–80.

16. Keller PM, Neff BJ, Ellis RW. Three major glycoprotein genes of varicella-zoster virus whose products have neutralization epitopes. J Virol. 1984; 52:293–297.

17. Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev. 2010; 23:202–217.

18. Sauerbrei A, Wutzler P. Serological detection of specific IgG to varicella-zoster virus by novel ELISA based on viral glycoprotein antigen. Clin Lab. 2009; 55:1–7.
19. Sauerbrei A, Wutzler P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol. 2006; 44:3094–3097.

20. Provost PJ, Krah DL, Kuter BJ, Morton DH, Schofield TL, Wasmuth EH, et al. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine. 1991; 9:111–116.

21. Krah DL, Cho I, Schofield T, Ellis RW. Comparison of gpELISA and neutralizing antibody responses to Oka/Merck live varicella vaccine (Varivax) in children and adults. Vaccine. 1997; 15:61–64.

22. Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007; 56(RR-4):1–40.
23. Gillet Y, Habermehl P, Thomas S, Eymin C, Fiquet A. Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (M-M-RvaxPro) and a varicella vaccine (VARIVAX) by intramuscular or subcutaneous routes at separate injection sites: a randomised clinical trial. BMC Med. 2009; 7:16.

24. Michalik DE, Steinberg SP, Larussa PS, Edwards KM, Wright PF, Arvin AM, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008; 197:944–949.

25. Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, et al. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002; 21:337–342.

26. Gershon AA, Steinberg SP, LaRussa P, Ferrara A, Hammerschlag M, Gelb L. Immunization of healthy adults with live attenuated varicella vaccine. J Infect Dis. 1988; 158:132–137.

27. Johnson C, Rome LP, Stancin T, Kumar ML. Humoral immunity and clinical reinfections following varicella vaccine in healthy children. Pediatrics. 1989; 84:418–421.

28. Watson B, Boardman C, Laufer D, Piercy S, Tustin N, Olaleye D, et al. Humoral and cell-mediated immune responses in healthy children after one or two doses of varicella vaccine. Clin Infect Dis. 1995; 20:316–319.

29. Saiman L, LaRussa P, Steinberg SP, Zhou J, Baron K, Whittier S, et al. Persistence of immunity to varicella-zoster virus after vaccination of healthcare workers. Infect Control Hosp Epidemiol. 2001; 22:279–283.

30. Kim YH, Hwang JY, Lee KM, Choi JH, Lee TY, Choi JS, et al. Seroepidemiologic survey of varicella-zoster virus in Korean adults using glycoprotein enzyme immuno assay and fluorescent antibody to membrane antigen test. Ann Dermatol. 2011; 23:39–43.

31. Zaia JA, Oxman MN. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977; 136:519–530.

32. Landry ML, Cohen SD, Mayo DR, Fong CK, Andiman WA. Comparison of fluorescent-antibody-to-membrane-antigen test, indirect immunofluorescence assay, and a commercial enzyme-linked immunosorbent assay for determination of antibody to varicella-zoster virus. J Clin Microbiol. 1987; 25:832–835.

33. Sauerbrei A, Färber I, Brandstädt A, Schacke M, Wutzler P. Immunofluorescence test for sensitive detection of varicella-zoster virus-specific IgG: an alternative to fluorescent antibody to membrane antigen test. J Virol Methods. 2004; 119:25–30.

34. Balfour HH Jr, Edelman CK, Dirksen CL, Palermo DR, Suarez CS, Kelly J, et al. Laboratory studies of acute varicella and varicella immune status. Diagn Microbiol Infect Dis. 1988; 10:149–158.

35. Kim SH, Lee HJ, Park SE, Oh SH, Lee SY, Choi EH. Seroprevalence rate after one dose of varicella vaccine in infants. J Infect. 2010; 61:66–72.

36. Breuer J, Schmid DS, Gershon AA. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough disease in vaccinees and to screen for susceptibility to varicella. J Infect Dis. 2008; 197:Suppl 2. S147–S151.

37. Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004; 23:132–137.

38. Lieberman JM, Williams WR, Miller JM, Black S, Shinefield H, Henderson F, et al. The safety and immunogenicity of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children: a study of manufacturing consistency and persistence of antibody. Pediatr Infect Dis J. 2006; 25:615–622.

39. de Ory F, Echevarría JM, Kafatos G, Anastassopoulou C, Andrews N, Backhouse J, et al. European seroepidemiology network 2: Standardisation of assays for seroepidemiology of varicella zoster virus. J Clin Virol. 2006; 36:111–118.

40. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984; 149:137–142.

41. Asano Y, Suga S, Yoshikawa T, Kobayashi I, Yazaki T, Shibata M, et al. Experience and reason: twenty-year follow-up of protective immunity of the Oka strain live varicella vaccine. Pediatrics. 1994; 94(4 Pt 1):524–526.

42. Johnson CE, Stancin T, Fattlar D, Rome LP, Kumar ML. A long-term prospective study of varicella vaccine in healthy children. Pediatrics. 1997; 100:761–766.

43. Kuter BJ, Weibel RE, Guess HA, Matthews H, Morton DH, Neff BJ, et al. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine. 1991; 9:643–647.

44. Watson B, Gupta R, Randall T, Starr S. Persistence of cell-mediated and humoral immune responses in healthy children immunized with live attenuated varicella vaccine. J Infect Dis. 1994; 169:197–199.

45. White CJ, Kuter BJ, Ngai A, Hildebrand CS, Isganitis KL, Patterson CM, et al. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr Infect Dis J. 1992; 11:19–23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download