Abstract
Purpose
Materials and Methods
Results
Conclusion
Figures and Tables
Fig. 1

Fig. 2

Fig. 3

Fig. 4

Table 1
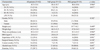
NaP, sodium phosphate; PEG, polyethylene glycol; N, number; BMI, body mass index; PC, previous colonoscopy.
All data are expressed as mean±standard deviation or number (percentage), as appropriate.
*p-value was calculated using the independent t-test.
†p-value was calculated using the chi-square test.
‡p-value was calculated using the Fisher-Freeman-Halton extension of Fisher's probability test.
Table 2
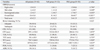
NaP, sodium phosphate; PEG, polyethylene glycol; N, number; OBPQS, Ottawa Bowel Preparation Quality Scale; CIR, cecal intubation rate; CIT, cecal intubation time; TIIR, terminal ileal intubation rate; TIIT, terminal ileal intubation time; WT, withdrawal time; TPT, total procedure time; PDR, polyp detection rate; ADR, adenoma detection rate.
All data are expressed as mean±standard deviation or number (percentage), as appropriate. The OBPQS was calculated by adding 0 to 4 points for each colon segment and 0 to 2 points for total fluid quantity in the entire colon. The scale has a range from 0 to 14. Adequate level of bowel cleansing was defined as a total OBPQS score of ≤4.
*p-value was calculated using the independent t-test.
†p-value was calculated using the chi-square test.
‡p-value was calculated using the Fisher-Freeman-Halton extension of Fisher's probability test.
Table 3
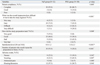
NaP, sodium phosphate; PEG, polyethylene glycol; N, number; cm, centimeter; VAS, visual analogue scale.
All data are expressed as in mean±standard deviation or number (percentage), as appropriate.
*p-value was calculated using the independent t-test.
†p-value was calculated using the chi-square test.
‡p-value was calculated using the Fisher-Freeman-Halton extension of Fisher's probability test.
Table 4
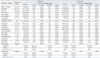
NaP, sodium phosphate; PEG, polyethylene glycol; WBC, white blood cells count; Hb., hemoglobin, Hct., hematocrit; Plt., platelet; FPG, fasting plasma glucose; BUN, blood urea nitrogen; Cr., creatinine; eGFR, estimated glomerular filtration rate; Na, sodium; K, potassium; Cl, chloride; TCO2, total carbon dioxide; Ca, calcium; IP, inorganic phosphorus; Mg, magnesium; AST, aspartate transaminase; ALT, alanine transaminase; Alb., albumin.
All data are expressed as mean±standard deviation or number (percentage), as appropriate.
*p-value was calculated using the paired t-test.
†p-value was calculated using the Wilcoxon signed rank test. Laboratory tests were conducted three times in all of the participants: i) baseline (the day of enrollment and allocation); ii) visit 1 (the day of colonoscopy and post-preparation); iii) visit 2 (1 week follow-up after colonoscopy). eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation: eGFR (milliliter per minute per 1.73 m2)=186.3×(serum creatinine)-1.154×(age)-0.203 (×0.742 if the individual was female).
Table 5

NaP, sodium phosphate; PEG, polyethylene glycol; WBC, white blood cells count; Hb., hemoglobin; Hct., hematocrit; Plt., platelet; FPG, fasting plasma glucose; BUN, blood urea nitrogen; Cr., creatinine; eGFR, estimated glomerular filtration rate; Na, sodium; K, potassium; Cl, chloride; TCO2, total carbon dioxide; Ca, calcium; IP, inorganic phosphorus; Mg, magnesium; AST, aspartate transaminase; ALT, alanine transaminase; Alb., albumin.
All data are expressed as mean±standard deviation or number (percentage), as appropriate. Laboratory tests were conducted three times in all of the participants: i) baseline (the day of enrollment and allocation); ii) visit 1 (the day of colonoscopy and post-preparation); and iii) visit 2 (1 week follow-up after colonoscopy). eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation: eGFR (milliliter per minute per 1.73 m2)=186.3×(serum creatinine)-1.154×(age)-0.203 (×0.742 if the individual was female).
*p-value was calculated using the independent t-test.
†p-value was calculated using the Fisher-Freeman-Halton extension of Fisher's probability test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download