Abstract
Purpose
For locally unresectable hepatocellular carcinoma (HCC) patients, concurrent chemoradiotherapy (CCRT) has been applied as a loco-regional treatment. After shrinkage of tumors in selected patients, surgical resection is performed. The aim of this study was to evaluate prognostic factors and long-term survivors in such patients.
Materials and Methods
From January 2000 to January 2009, 264 patients with HCC were treated with CCRT (45 Gy with fractional dose of 1.8 Gy), and intra-arterial chemotherapy was administered during radiotherapy. Eighteen of these patients (6.8%) underwent hepatic resection after showing a response to CCRT. Cases were considered resectable when tumor-free margins and sufficient remnant volumes were obtained without extrahepatic metastasis. Prior to operation, there were six patients with complete remission, 11 with partial remission, and six with stable disease according to modified Response Evaluation Criteria in Solid Tumors.
Results
In pathologic review, four patients (22.2%) showed total necrosis and seven patients (38.9%) showed 70-99% necrosis. A high level of necrosis (≥80%) was correlated with low risk for extrahepatic metastasis and long-term survival. In univariate analyses, vessel invasion and capsular infiltration were significantly correlated with disease free survival (DFS) (p=0.017 and 0.013, respectively), and vessel invasion was significantly correlated with overall survival (OS) (p=0.013). In multivariate analyses, capsule infiltration was a significant factor for DFS (p=0.016) and vessel invasion was significant for OS (p=0.015).
Hepatocellular carcinoma (HCC) is a major disease in Asian countries. Global cancer statistics showed this disease as the third most common cause of cancer-related deaths worldwide.1 Since HCC is unresectable in the majority of patients at the time of initial visiting, patients are frequently referred to non curative treatments. Based on the results of randomized clinical trials, sorafenib has become the first-line therapy for advanced Barcelona Clinic Liver Cancer (BCLC) stage C HCC.2,3 However, BCLC stage C represents a variety of disease features, including metastasis, portal vein invasion, and performance 0-2. In this stage, National Comprehensive Cancer Network guidelines suggest that radiotherapy (RT) can be considered as an alternative to ablation/embolization techniques for unresectable HCC with category 2B level evidence. Lee and Seong4 reviewed the optimal indications for RT according to the BCLC staging system and the application of various RT modalities and prescription according to the extent of disease. Combining RT with radiosensitizers such as concurrent chemoradiotherapy (CCRT) is an also attractive strategy to increase the therapeutic ratio for these patients.5
Several studies reported downstaging or total necrosis of the tumor after non-surgical modalities, including transcatheter arterial chemoembolization (TACE), hepatic artery chemo-infusion, combined systemic chemotherapy, external radiation, and radioimmunotherapy.6,7,8,9 Although tumor shrinkage was sufficient to allow salvage liver resection following therapy in only a small proportion of patients, the potential for curative therapy in responders also gives hope to patients with unresectable HCC.
In our institute, patients with locally advanced BCLC stage C were treated with CCRT when surgery was not feasible due to portal vein tumor thrombosis or inadequate non-tumor liver volume.10 Among them, some patients underwent surgical resection after showing a response to CCRT. In this study, we evaluated prognostic factors and long-term survival in patients who underwent hepatic resection after CCRT.
From January 2000 to January 2009, 264 patients with locally advanced HCC were treated with external beam RT and concurrent intra-arterial (iA) chemotherapy (CTx) when surgery was not feasible due to portal vein tumor thrombosis or inadequate non-tumor liver volume. In some patients, CCRT showed a favorable response and eighteen of these patients (6.8%) underwent hepatic resection after multidisciplinary HCC conference. All patients provided informed written consent for participation in this study, which was approved by our Institutional Review Board. Table 1 shows the clinical characteristics of these patients. The median follow-up of the 18 patients was 40 months.
Diagnosis of HCC was based on either pathologic confirmation or radiologic findings with an elevated serum α-fetoprotein (AFP). If computed tomography (CT), magnetic resonance imaging (MRI), or arteriography detected a cirrhotic liver with a hepatic mass larger than 2 cm (which suggests HCC) accompanied by AFP elevated more than 200 ng/mL, a biopsy was not necessary for HCC diagnosis. In order to be selected as a candidate for the CCRT protocol, patients needed to have an Eastern Cooperative Oncology Group performance status of 0-2 and adequate liver function [indocyanine green R15 (ICG R15) <30% and Child-Pugh class A or B]. Patients with tumors of diffuse or multifocal bilobal involvement were excluded to avoid whole-liver irradiation.
All of the 18 patients were treated with three-dimensional conformal radiotherapy. The gross tumor volume (GTV) was defined as the radiographically abnormal areas detected in the CT or MRI images, and a minimum of 5 mm around the GTV was added to determine the clinical target volume (CTV). The range of diaphragmatic movement was measured via fluoroscope in order to incorporate cranio-caudal movement of the liver into defining the internal target volume (ITV). In defining planning target volume (PTV), an additional 5 mm for set-up error was added to ITV. A total of 45 Gy was prescribed to the isocenter in 25 fractions (1.8 Gy/fraction) over 5 weeks using a 6-megavolt (MV) or 10-MV linear accelerator with the intention to deliver 95% of the prescribed dose encompassing the PTV around the CTV.10
Continuous-infusion hepatic arterial 5-fluorouracil (5-FU) at a dose of 500 mg/day was delivered during the first and fifth weeks of RT. One month after CCRT, hepatic arterial 5-FU (at a dose of 500 mg/m2 for 5 hours on Days 1-3) and cisplatin (at a dose of 60 mg/m2 for 2 hours on Day 2) were administered every 4 weeks for 3 to 12 cycles according to tumor response.
To evaluate operability, all patients underwent preoperative liver function tests, Child-Push score reassessment, and measurement of the ICG R15. The extent of resection was determined according to the ICG R15 and gross findings of the liver during a laparotomy. ICG R15 <10% was regarded as having no impaired liver function.11 For our hospital, cases were considered resectable if there was no extrahepatic metastasis on preoperative imaging studies, and if adequate tumor-free margins and sufficient remnant volumes were obtained.12 The resectability of tumors was finally determined at multidisciplinary HCC conferences before and after CCRT.
Two months after the completion of CCRT and at short-interval (3-6 months) follow-up, abdominal/pelvic CT was used to evaluate radiologic tumor response. Modified Response Evaluation Criteria in Solid Tumors criteria were used to evaluate responses to CCRT.13 Serum AFP and protein induced by vitamin K absence (PIVKA-II) were measured at diagnosis and 2 months after CCRT for evaluation of chemical response, as well as at 3-6 months during follow-up, to detect recurrence. For chemical response, complete remission (cCR) was defined as normalization of tumor marker, partial response (cPR) as at least a 50% decrease from the pretreatment level, progressive disease (cPD) as >25% increase of tumor marker from the pretreatment level, and stable disease (cSD) as any changes that do not meet other criteria.
A pathologist evaluated the resected tumor for evidence of gross and histologic necrosis after serial sectioning using a standard protocol (10 mm slice thickness). Slides were prepared using routine hematoxylin and eosin staining. The irradiated lesion was examined for the presence of viable neoplastic tissue, capsular invasion, microscopic vascular invasion, tumor size, tumor multiplicity, and resection margins, and a pathologist quantitatively evaluated the percentage of necrosis.
Patient survival was measured from the beginning of radiotherapy. Overall survival (OS) and disease free survival (DFS) were analyzed using proportional hazards (Cox) regression. The median survival time was estimated using Kaplan-Meier method.
Radiologic and chemical responses were determined 2 months after the completion of CCRT and immediately before surgical resection. Table 2 shows the results of the treatment. Two months following CCRT, two (11.1%), 13 (72.2%), and three (16.7%) patients achieved radiological CR, PR, and SD, respectively. Immediately before the operation, six (33.3%), 11 (61.1%), and one (5.6%) patients achieved CR, PR, and SD, respectively. Among the nine patients whose AFP level was above 200 ng/mL at diagnosis, cCR and cPR were achieved in two (11%) and seven (39%) patients at 2 months from CCRT, and cCR, cPR, or cPD were shown in five (28%), three (17%), and one (5.5%) patients immediately before the operation, respectively. Among the 13 patients with elevated PIVKA-II above 250 ng/mL, cCR, cPR, and cPD were observed in six (33%), five (28%), and two (11%) patients 2 months after CCRT. The cCR, cPR, and cPD were observed in seven (39%), five (28%), and one (5.5%) patients immediately before the operation, respectively.
The median time interval from CCRT to surgical resection was 6.2 months (range 1-21 months). The numbers of patients who received right lobectomy, left lobectomy, central lobectomy, and bisegmentectomy were 12, four, one, and one, respectively. Table 3 shows reports from the pathologic review. Single and multiple tumors were found in 10 (56%) and eight (44%) patients, respectively, and four patients (22%) had tumors larger than 10 cm in diameter. Vessel invasion and capsular infiltration each were confirmed in nine patients (50%), and close resection margin (≤1.0 cm) was detected in seven patients (39%). Four patients (22%) had total necrosis, seven patients (39%) had 70-99% necrosis, and less than 70% necrosis was observed in seven patients (39%). Fig. 1 shows a case of 51-year-old male patient with a large HCC who achieved a pathologic CR (16 cm→10 cm, 100% necrosis) following CCRT. We performed a subgroup analysis to examine the correlation between pathologic findings and known biomarkers, such as AFP and PIVKA-II. We found that elevated levels of preoperative AFP (≥200 ng/mL) were a significant predictive factor for large tumor size (p=0.005), and elevated preoperative PIVKA-II (≥250 ng/mL) levels were able to significantly predict of the capsular invasion (p=0.02), as well as the degree of necrosis above 80% (p=0.009).
The number of patients with intrahepatic metastasis was six (33.3%) and distant metastasis was eight (44.4%). For distant metastases, lung was the most common site, followed by lymph nodes, pleural/omental seeding, and the brain. In the analysis of patterns of treatment failure (Table 4), high necrosis rate (≥80%) was associated with a low incidence of extrahepatic metastasis (p=0.02).
The 3-year OS and DFS were 59.3% and 33.3%, respectively, and the median OS and DFS were 61.8 months and 24.1 months, respectively. In univariate analyses, vessel invasion was significant for decreased OS (p=0.013), while other variables including capsule invasion, positive resection margin, necrosis less than 100%, and tumor size ≥10 cm showed no significant correlation with OS. Findings of vessel invasion and capsular infiltration were significant for decreased DFS (p=0.017 and 0.013). In multivariate analyses, vessel invasion was a significant factor for OS (p=0.015), and capsule infiltration was significantly correlated with DFS (p=0.016) (Table 5).
Table 6 shows clinical and pathologic factors of the three patients who had no evidence of disease for long-term (>5 years) at the time of surgical treatment. The stage of these patients was III or VI-A, and the range of tumor size was 7-16 cm. Because two of them had portal vein thrombosis and another had extensive multi-lobar tumors, although the tumor size was not huge (7 cm), they did not undergo surgery as the first treatment. After CCRT, these patients showed good response (CR or PR) and the disease was converted to operable stage. Before surgical treatment, preoperative levels of tumor markers (AFP and PIVKA-II) were normalized in all patients. After operation, one radiologic CR with 100% necrosis, and two PRs with 90-100% necrosis were achieved, and neither vascular nor capsular invasion was detected in pathologic findings.
Surgical resection of tumors gives the best opportunity of a cure for patients with HCC. However, the resectability of HCC is low at initial diagnosis (10-30%). Among the unrectable patients, patients with poor general condition or decompensated liver disease could be candidates for tumor downstaging and salvage liver resection. The decision making for the unresectability of HCC include a large tumor volume with insufficient normal hepatic mass after liver resection, extensive and multifocal bilobar tumors, extrahepatic spread of the disease, and tumors with main portal vein/hepatic vein/inferior vena cava tumor thrombus. For Asian patients, 80-90% of cases of HCC develop in cirrhotic livers. Although a normal liver can permit up to 80% of resection of functional liver volume, cirrhotic liver is a major risk factor for the development of postoperative liver insufficiency, failure, and mortality. Even minor resection of a cirrhotic liver can result in post-resection liver failure. Therefore, the resection of liver that should be safely monitored according to the degree of cirrhosis, the functional reserve, and the regenerative response to the surgical intervention. A main vessels tumor thrombus usually occurs as a late complication of large or diffuse HCC, and is associated with a poor median survival of 2 months. Such tumors are therefore traditionally considered unsuitable for partial hepatectomy. However, the decision on whether or not a tumor is resectable is still largely subjective. This is a common criticism of studies on salvage surgery following tumor downstaging. In radiation oncologists' view, "locally advanced hepatocellular carcinoma" is defined as disease not acceptable to surgical resection or immediate liver transplantation. The extent of disease could be defined by BCLC(B) intermediate stage or BCLC (C) advanced stage without extrahepatic spread except for regional lymph node involvement.14 In our study, the resectability of tumors was determined at multidisciplinary HCC conferences before and after CCRT.
Recent reports have revealed that RT can be delivered at a high dose to the focal liver with acceptable complications.15,16,17,18 Global experience with the use of a combination of RT and radiosensitizers in HCC treatment is limited.5 CCRT has shown excellent tumor responses with prolonged survival in selected series of HCC patients.19,20,21 However, there is a still risk of radiation induced toxicity. We previously reported that several factors, such as tumor size, pre-RT ICG-R15, and Child-Pugh class could be useful for predicting radiation hepatotoxicity in HCC patients treated with RT.22,23,24 Han, et al.10 reported the therapeutic effect of concurrent fluorouracil (5-FU) and RT followed by iA CTx in patients with locally advanced HCC with portal vein thrombosis. In most tumor sites, the majority of experience with radiation sensitizers is through systemically delivered agents. In HCC, however, there has been more experience with hepatic arterial regional chemotherapy delivered with RT.25,26 The rationale for selective radiosensitization is that the exposure of non-tumor normal tissues to radiosensitizer is reduced, improving the therapeutic ratio and potentially leading to increased doses to the focal HCC, thereby increasing the tumor response. Regional perfusion of drugs with high hepatic extraction rates and short serum half-lives allow much higher drug doses to be delivered to focal HCCs. iA CTx alone or with TACE, takes advantage of this selective blood supply to HCC versus normal liver. In our study, all patients had Child Pugh A liver function. The objective response rate was 45% (18 of 40 patients). The actuarial 3-year OS rate was 24.1%, and the median survival time was 13.1 months from the start of RT. The results were better than a similar group of patients with a 4-6 months median survival.10 Following this treatment, the shrunken tumor as well as compensatory hyperplasia of the non-tumor liver allowed for surgical resection in a small portion of patients. According to the initial response after localized CCRT, patients with good response showed significantly improved survival (p=0.033). The median survival time of responders was 19.9 months, compared with 11.4 months for non-responders. In our study, 264 patients with HCC were treated with CCRT, and 18 patients (6.8%) underwent hepatic resection. These patients showed better 3-year OS (59.3%) than our previous results of our CCRT study (24.1%). However, this result was restricted from highly selected patients.
Tang, et al.6 reported residual disease in 69.7% of the surgical specimens resected after combination treatments. Majno, et al.7 described that downstaging or total necrosis of the tumor after TACE occurred in 62% of liver resected patients. A total necrosis after TACE was reported at 24 patients (50%). In liver transplantation cases, downstaging of tumors >3 cm (54% of patients) and total necrosis (28% of patients) were associated with superior disease-free survival (p=0.01 and p=0.03). Huang, et al.8 also mentioned that only a small portion of tumors showed complete necrosis after TACE. Total necrosis (100%) of tumor was observed in 11.1% of 117 patients. Fan, et al.9 showed that complete tumor necrosis occurred in 11 patients (16.9%). There were 6 patients who had undergone five TACE treatments each, but their percentage of necrotic area was only 60-90%. Choi, et al.12 reported six patients (12%) who had undergone TACE followed by RT showed total necrosis.
Since the waiting list is long and patients may drop out owing to tumor progression, radiation therapy may have a role in selected patients. There are several studies on using radiation therapy as a bridge to liver transplant. Sandroussi, et al.27 reported their experience with treating 10 patients with conformal radiotherapy as a novel bridge to liver transplantation. There was no complete tumor necrosis and 4 patients demonstrated a pathological response with the degree of necrosis ranging from 40% to 90%. The lack of complete necrosis may be related to the sublethal radiation dose, owing to concerns about radiation-induced liver disease. In a recent case report, the explant demonstrated complete histological necrosis of the lesion after external-beam radiotherapy in patients who were candidates for liver transplantation.28 O'Connor, et al.29 also reported that the explant pathology revealed no viable tumor in three of the 11 tumors they studied, resulting in a complete response rate of 27%. Our pathological review of resected specimens after CCRT showed a similar rate of complete necrosis (22.2%), and 7 patients (38.9%) showed 70-99% necrosis. For the patterns of failure, the number of patients with intrahepatic metastases was six (33.3%), and high necrosis (≥80%) indicated a low risk for extrahepatic metastasis. These results demonstrate that using radiation therapy as a bridge to liver transplant provided a clear advantage in terms of local tumor control. In several studies, vascular invasion, multiple tumors, a high value of AFP, and cirrhosis were shown to be significant prognostic factors for survival after resection of huge HCC.30,31,32 Tumor size is also an important predictor for disease free survival and overall survival after resection in patients with HCC. When the tumor size is bigger, the incidence of vascular invasion and intrahepatic metastasis also increases. Choi, et al.12 evaluated the surgical outcomes of 50 patients with ≥10 cm HCC. The 5 year-overall survival was 40.2%, and a multivariate analysis showed that the single nodular tumor type was the only significant prognostic factor for survival. These patients also had a more incidence of high AFP >1000 IU/mL, microscopic vascular invasion, and advanced stage tumors than patients with smaller tumors. Mean tumor size of the present study was 10.7 cm before CCRT, and histologic prognostic factors such as vessel invasion, capsular infiltration, resection margin positivity, and tumor size were also analyzed in this study. Vessel invasion was significant for OS and DFS, and capsular infiltration was significant for DFS in univariate analysis. In multivariate analyses, vessel invasion was a significant factor for OS, and capsule infiltration was significantly correlated with DFS. Patients who remained without evidence of disease for a long time also showed favorable pathologic findings (Table 6), and these results were similar to surgical studies.
In our previous results, we suggested CCRT followed by iA CTx to be a useful modality.10,33,34 This study described that CCRT could also achieved downstaging and conversion to operable status in selected patients with unresectable HCC. Our data suggest that there is hope for long-term survival through surgical resection. However, this study has limitations of a small sample size (18 patients) and the risk of type II error is high. Notwithstanding, the results are similar to those of other studies on TACE followed by downstaging surgery or other results for surgical outcomes for large HCC.9,12 Our data suggest that long-term survival may be possible in total necrosis, as well as capsular and vessel invasion negative patients. Therefore, tailored strategies according to the pathologic findings after downstaging surgery after CCRT may be effective. However, more clinical experience and prospective studies are needed to determine the role of downstaging by CCRT. In selected patients, CCRT showed favorable response and locally advanced HCC converted into resectable tumor after CCRT. Long-term survivors showed the pathological features of near total necrosis, as well as negative capsule and vessel invasion.
Figures and Tables
Fig. 1
A 51-year-old male diagnosed with 16 cm HCC in the left lobe, cT4N0M0, PVTT (+), iAFP 89.3 ng/mL; initially treated with TACE (A). Tumor mass slightly decreased in size, 14.6×9.1 cm, compared to pre-RTx CT images, at which it measured 16.5×10.5 cm. Focal necrosis and radiation induced changes are shown (B). Partial response shown in C (preop image 10×9 cm, preop AFP 2.0 ng/mL). Concurrent chemoradiotherapy (CCRT) 45 Gy with intra-arterial chemotherapy (iA CTx), followed by iA CTx 6 cycles (D). Left lobectomy 15 months after CCRT, 100% necrosis, negative resection margin, vascular invasion, and capsule invasion (E). He remained disease free, until now, 69 months after surgical resection. HCC, hepatocellular carcinoma; iAFP, α-fetoprotein at diagnosis; TACE, transcatheter arterial chemoembolization; PVTT, portal vein tumor thrombosis; RTx, radiotherapy.
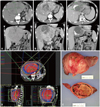
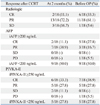
Table 6
Clinical Characteristics of Patients Who Remained without Evidence of Disease for >5 Years

S/A, sex/age; CRT, chemoradiation; OP, operation; AFP, α-fetoprotein; PIVKA, protein induced by vitamin K absence; TtOP, time interval to operation; RM, resection margin; VI, vascular invasion; CI, capsular infiltration; DFS, disease free survival; iA CTx, intra-arterial chemotherapy; CR, complete remission; PR, partial response; RT, radiotherapy.
ACKNOWLEDGEMENTS
This study was supported by the faculty research grant from Yonsei University College of Medicine (6-2011-0077) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0004085).
Notes
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

2. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

3. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.

4. Lee IJ, Seong J. Radiotherapeutic strategies in the management of hepatocellular carcinoma. Oncology. 2011; 81:Suppl 1. 123–133.

5. Lee IJ, Seong J. Radiosensitizers in hepatocellular carcinoma. Semin Radiat Oncol. 2011; 21:303–311.

6. Tang ZY, Yu YQ, Zhou XD, Ma ZC, Lu JZ, Liu KD, et al. Cytoreduction and sequential resection: a hope for unresectable primary liver cancer. J Surg Oncol. 1991; 47:27–31.

7. Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997; 226:688–701.

8. Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl). 2000; 113:446–448.
9. Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998; 15:674–678.

10. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003.

11. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298–304.

12. Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009; 198:693–701.

13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.

14. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999; 19:329–338.

15. Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990; 19:1041–1047.

16. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–122.

17. Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999; 43:393–397.

18. Kim YI, Park HC, Lim do H, Park HJ, Kang SW, Park SY, et al. Changes of the liver volume and the Child-Pugh score after high dose hypofractionated radiotherapy in patients with small hepatocellular carcinoma. Radiat Oncol J. 2012; 30:189–196.

19. Han KH, Lee JT, Seong J. Treatment of non-resectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2002; 17:Suppl 3. S424–S427.

20. Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005; 23:8739–8747.

21. Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000; 18:2210–2218.

22. Yoon HI, Koom WS, Lee IJ, Jeong K, Chung Y, Kim JK, et al. The significance of ICG-R15 in predicting hepatic toxicity in patients receiving radiotherapy for hepatocellular carcinoma. Liver Int. 2012; 32:1165–1171.

23. Lee IJ, Seong J, Shim SJ, Han KH. Radiotherapeutic parameters predictive of liver complications induced by liver tumor radiotherapy. Int J Radiat Oncol Biol Phys. 2009; 73:154–158.

24. Lee IJ, Seong J, Shim SJ, Han KH, Chon CY. [Reappraisal of risk factors predicting liver complications from radiotherapy for hepatocellular carcinoma]. Korean J Hepatol. 2006; 12:420–428.
25. Wellwood JM, Cady B, Oberfield RA. Treatment of primary liver cancer: response to regional chemotherapy. Clin Oncol. 1979; 5:25–31.
26. Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992; 69:920–924.

27. Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010; 23:299–306.

28. Wigg A, Hon K, Mosel L, Sladden N, Palumbo K. Down-staging of hepatocellular carcinoma via external-beam radiotherapy with subsequent liver transplantation: a case report. Liver Transpl. 2013; 19:1119–1124.

29. O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012; 18:949–954.
30. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457.

31. Nagano Y, Tanaka K, Togo S, Matsuo K, Kunisaki C, Sugita M, et al. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg. 2005; 29:66–71.

32. Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007; 14:2817–2823.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download