Abstract
Purpose
Despite significant improvements in surgery, anesthesia, and postoperative critical care, the postoperative mortality rate of ruptured abdominal aortic aneurysm (RAAA) has remained at 40% to 50% for several decades. Therefore, we evaluated factors associated with the postoperative mortality of RAAA.
Materials and Methods
From January 1999 to December 2008, a retrospective study was performed with 34 patients who underwent open repair of RAAA. The preoperative factors included age, sex, smoking, comorbidities, serum creatinine, hemoglobin, shock, pulse rate, and time from emergency room to operation room. The intraoperative factors included blood loss, transfusion, aortic clamping site and time, aneurysmal characteristics, rupture type, graft type, hourly urine output (HUO), and operative time. The postoperative factors included inotropic support, renal replacement therapy (RRT), reoperation, bowel ischemia, multiple organ failure (MOF), and intensive care unit stay. The 2-day and the 30-day mortality rates were analyzed separately.
Results
The 2-day and the 30-day mortality rates were 14.7% and 41.2%, respectively. On univariate analysis, shock, transfusion, HUO, inotropic support and MOF for the 2-day mortality and serum creatinine, transfusion, aortic clamping site, HUO, inotropic support, RRT and MOF for the 30-day mortality were statistically significant. On multivariate analysis, shock, inotropic support and MOF for the 2-day mortality and aortic clamping site, RRT and MOF for the 30-day mortality were statistically significant.
The postoperative mortality rate of elective surgery for non-ruptured abdominal aortic aneurysm (AAA) has been reported to be less than 5%.1,2 And, it has been reported that if the diameter of AAA is more than 5.5 cm, the risk of rupture of AAA is increased; if the diameter of AAA is more than 6.5 cm, the risk of rupture of AAA is greatly increased.3 Despite significant improvements in surgery, anesthesia, and postoperative critical care, the mortality rate of ruptured AAA (RAAA) has not changed substantially over the last two decades.4,5 According to most reports,3,6,7 the postoperative mortality rate of RAAA has remained at 40-50%, reaching 75-90% if deaths occurring before patients' arrival at the hospital are included. The primary causes of death in patients undergoing emergency repair of RAAA, in most reports, are massive hemorrhage, cardiorespiratory dysfunction, and acute renal failure (ARF).8 With respect to the high mortality rate of RAAA, Scarcello, et al.4 asserted that time before shock was the most important predictor of the postoperative mortality for RAAA, while Davies, et al.8 reported postoperative renal replacement therapy (RRT) as an independent risk factor of RAAA, and Halpern, et al.9 argued that loss of consciousness, preoperative hemoglobin level, preoperative and intraoperative systolic blood pressure, intraoperative urine output, intraoperative blood loss, and intraoperative transfusion were risk factors of the mortality of RAAA. Similar to these, various authors have reported different factors as risk factors of the postoperative mortality of RAAA.4,8,9 Therefore, we evaluated patients who underwent surgery of RAAA to define factors associated with the postoperative mortality of RAAA. This research was approved by the Institutional Review Board of our institute.
A retrospective review of 34 patients, who were diagnosed with RAAA and underwent open repair at our hospital between January 1999 and December 2008, was performed using their medical records. There were 246 patients who were diagnosed with AAA during this period. Among 246 patients, 43 patients were diagnosed with RAAA, but we excluded 9 patients that had expired in the emergency room (ER) before they underwent repair of RAAA. All open repairs of RAAA for the 34 patients during this period had been performed by surgical staff with vascular specialty and anesthesia staff with anesthesia specialty. To maintain hemodynamic status prior to surgery, we made an effort to conduct prompt diagnosis and recovery from a shock state by fluid resuscitation and transfusion with plasma components in principle. We divided factors associated with the postoperative mortality of RAAA into preoperative, intraoperative, and postoperative factors. The preoperative factors included age, sex, history of smoking, comorbidities [ischemic heart disease (IHD), diabetes mellitus (DM), hypertension, stroke, chronic renal failure (CRF), chronic obstructive pulmonary disease (COPD)], serum creatinine, hemoglobin, shock, pulse rate, and time from ER to operation room (OR). Shock meant circulatory shock defined as a systolic blood pressure <80 mm Hg upon hospitalization before the procedure.10 The intraoperative factors included blood loss, transfusion, aortic clamping site, aortic clamping time, characteristics of AAA (site, etiology, and shape) by standards for reporting,11 rupture type,11,12 involvement of the internal iliac artery (IIA), type of rupture, type of graft, sacrification of the IIA, hourly urine output (HUO), and operation time. We checked the amount of blood loss by counting the amount of blood collected in a suction bottle during the operation. Rupture types were defined according to the Fitzgerald classification where A type included intramural bleeding or a small hematoma, B type included a hematoma below the renal arteries and including the pelvis, C type comprised a hematoma extending above the renal arteries and into the pelvis, and D type comprised free blood in the peritoneal cavity.12 The postoperative factors included inotropic support, RRT, reoperation, bowel ischemia, multiple organ failure (MOF), and intensive care unit (ICU) stay. MOF involved renal failure, hepatic failure, pulmonary failure, and heart failure. The mortality rate was divided into postoperative 2-day and 30-day mortality rates. We also analyzed the 2-day and the 30-day mortality rates separately. There was no patient that received endovascular abdominal aneurysm repair (EVAR) for RAAA. Among 34 patients, 29 patients had been referred from other hospitals to the ER of our hospital for repair of RAAA, and 5 patients had been diagnosed in RAAA at the ER of our hospital without previous diagnosis of AAA. So, we have no information about AAA before rupture for 34 patients. Statistical analysis was done by Chi-square test, Fisher's exact test, and logistic regression using the SAS system for Windows, version 8. At first, we performed univariate analysis for the association of postoperative mortality and factors. Factors found to be significant on univariate analysis were entered into a multivariate analysis. p-values <0.05 were considered statistically significant. Data were presented as mean±standard deviation.
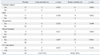
For the 34 patients who underwent open repair of RAAA, the mean age was 70.0±7.8 years. The sexual distribution was 27 male patients (79.4%) and 7 female patients (20.6%) (Table 1). Comorbidities consisted of hypertension in 23 patients (67.6%), DM in 5 patients (14.7%), and IHD in 4 patients (11.8%) (Table 1). The level of preoperative serum creatinine (mg/dL) was 1.80±1.13, the level of preoperative hemoglobin (g/dL) was 10.19±2.18, the time from ER to OR (min) was 133.52±39.54, the amount of intraoperative blood loss (L) was 4.63±3.86, the amount of intraoperative transfusion [packed red blood cells (PRBC)] (u) was 12.18±9.00, the intraoperative aortic clamping time (min) was 78.53±30.45, the HUO (mL) was 107.76±57.16, and the operation time (min) was 294.12±68.23 (Table 1 and 2). The main reason of massive bleeding during surgery was coagulopathy, and the other reasons included intraperitoneal rupture before aorta clamping and anastomosis site bleeding. The postoperative mortality rates were 14.7% (5 patients) for the 2-day mortality rate and 41.2% (14 patients) for the 30-day mortality rate (Table 1, 2 and 3).
On univariate analysis of the association of the 2-day mortality and factors associated with the postoperative mortality of RAAA, preoperative shock was statistically significant (p=0.038) (Table 1), and 4 out of 13 patients with shock expired. All preoperative factors except for shock were statistically insignificant (p>0.05) (Table 1). Among intraoperative factors, intraoperative transfusion was statistically significant (p=0.031) (Table 2). In total, 5 of 18 patients with transfusion (u) of more than 10 expired. HUO was statistically significant (p=0.019) (Table 2), and 4 of the 10 patients with HUO (mL) of less than 100 expired. Blood loss, aortic clamping site, aortic clamping time, characteristics of AAA, type of rupture, Fitzgerald classification, sacrification of IIA, type of graft, and operation time were statistically insignificant (p>0.05) (Table 2). Rupture types of all 34 patients were retroperitoneal ruptures (Table 2). According to Fitzgerald classification, 11 of 34 patients were B type, and the other 23 patients were C type (Table 2). Among postoperative factors, inotropic support was statistically significant (p=0.022) (Table 3), and 5 of 17 patients with inotropic support expired. Also, 5 of 14 patients with MOF expired, and MOF was found to be statistically significant (p=0.007) (Table 3). RRT, reoperation, bowel ischemia, and ICU stay were statistically insignificant (p>0.05) (Table 3).
On multivariate analysis of the association of the 2-day mortality and factors found to be significant on univariate analysis, preoperative shock (p=0.049), postoperative inotropic support (p=0.002) and postoperative MOF (p=0.001) were statistically significant (Table 4).
On univariate analysis of the association of the 30-day mortality and factors associated with the postoperative mortality of RAAA, among preoperative factors, serum creatinine was statistically significant (p=0.030) (Table 1). Seven of 10 patients with serum creatinine (mg/dL) of more than 2.0 expired. All preoperative factors except for serum creatinine were statistically insignificant (p>0.05) (Table 1). Among the intraoperative factors, transfusion was statistically significant (p=0.012) (Table 2). Eleven of 18 patients with transfusion (u) of more than 10 expired. As 6 of 7 patients with suprarenal aortic clamping expired, aortic clamping site was found to be statistically significant (p=0.011) (Table 2). Also, 8 of 10 patients with HUO (mL) of less than 100 expired, and HUO was statistically significant (p=0.006) (Table 2). All other intraoperative were statistically insignificant (p>0.05) (Table 2). Among the postoperative factors, inotropic support was statistically significant (p=0.005) (Table 3). Eleven of 17 patients with inotropic support expired. Eight of 11 patients with RRT expired, and RRT was statistically significant (p=0.012) (Table 3). All 14 patients with MOF expired, and MOF was statistically significant (p<0.001) (Table 3). All other postoperative factors were statistically insignificant (p>0.05) (Table 3).
On multivariate analysis of the association of 30-day mortality and factors found to be significant on univariate analysis, intraoperative aortic clamping site (p=0.004), postoperative RRT (p=0.001) and postoperative MOF (p<0.001) were statistically significant (Table 4).
The causes of death for the 5 patients that expired within 2 days after open repair of RAAA were bleeding in 3 patients and acute myocardial infarction (MI) in 2 patients (Table 5). The causes of death for the 14 patients that expired within 30 days after open repair of RAAA were bleeding in 4 patients, acute MI in 4 patients, acute respiratory distress syndrome in 3 patients, ARF in 2 patients, and stroke in 1 patient (Table 5). We diagnosed acute MI as the cause of death according to the symptoms of the patients, as well as changes in rhythms on electrocardiography (ECG) and elevations of cardiac enzymes. No patient died of the abdominal compartment syndrome.
The postoperative mortality rate of RAAA has been reported as 40-50% in most reports.2-6,13,14 Despite several advances in diagnostic imaging as well as intraoperative and postoperative critical care, the postoperative mortality rate of RAAA has not changed substantially over the last two decades.5,13 According to Korean reports, the postoperative mortality rates of RAAA have been reported as 36% by Kim, et al.,2 17.1% by Cho, et al.,3 and 36.8% by Beah, et al.14 However, Cho, et al.3 explained that the reason their mortality rate was relatively low was because there was no free peritoneal ruptures of AAA.
In our study, we divided postoperative mortality into 2-day and 30-day mortality rates, and analyzed these separately. Our results showed that the 2-day mortality rate was 14.71%, and the 30-day mortality rate was 41.18%. These results were comparable to those of other reports.2-6,13,14 In our study, we performed separate analysis of the 2-day mortality to find factors directly associated with postoperative mortality during the earlier and more critical period after open repair of RAAA, as we conjectured that 2-day mortality would more directly reflect the influences of factors, such as preoperative status and intra- and post-operative bleeding, on postoperative mortality in earlier periods than the 30-day mortality. Also, we thought that 30-day mortality would better reflect the influences of factors on postoperative mortality in the recovery period than the 2-day mortality rate. In our study, preoperative shock, postoperative inotropic support and postoperative MOF were significantly related with 2-day mortality, suggesting that the patients who could not recover from shock during surgery died. As for the 30-day mortality, intraoperative aortic clamping site, postoperative RRT and postoperative MOF demonstrated significant correlation. This suggested that even though the patients survived the shock of the emergency surgery, renal dysfunction, probably caused by renal ischemia, became the main cause of death. Accordingly, we think that there were meaningful differences in the factors related with mortality between 2-day and 30-day mortality.
Much research has been performed in an attempt to define factors associated with the mortality of RAAA.2,3,6,8,9,13-19 In the literature,2,3,6,8,9,13-19 age, sex, comorbidities (CRF, COPD, IHD etc.), hemodynamic status, cardiac arrest, postoperative care, complication, volume of surgeons, and specialty are reported to be significantly associated with the postoperative mortality of RAAA. Cho, et al.3 suggested that intraoperative transfusion and renal dysfunction, such as intraoperative oliguria (<200 mL), consistent anuria, and postoperative increase of serum creatinine (>2.0 mg/dL), were significant risk factors associated with the postoperative mortality of RAAA. Davies, et al.8 reported that RRT was a significant risk factor of the postoperative mortality of RAAA, and Beah, et al.14 suggested that preoperative cardiac arrest was a significant risk factor of the postoperative mortality of RAAA. Rutledge, et al.15 reported that age was an important risk factor, as a mortality rate of more than 50% for patients older than 65 years was recorded in research of 1480 patients. Boyle, et al.17 suggested that age, ischemic electrocardiography (ECG), hemoglobin, serum creatinine, and loss of consciousness were preoperative risk factors. Sinicrope, et al.19 asserted that postoperative renal failure was the most important predictive factor of mortality. In such situations, the causes of renal failure are mostly a decrease in renal perfusion and renal ischemic change due to hypotension and decreases in systemic blood volume from massive bleeding, and other causes could include the use of contrast dye and intraoperative suprarenal aortic clamping.3
In our study, we divided the factors associated with the postoperative mortality of RAAA into preoperative, intraoperative, and postoperative factors. On multivariate analysis, preoperative shock, postoperative inotropic support and postoperative MOF were significantly associated with the postoperative 2-day mortality, while intraoperative aortic clamping site, postoperative RRT, and postoperative MOF were significantly associated with the postoperative 30-day mortality. During both the earlier, more critical period (<2 days) and the recovery period (<30 days) after open repair of RAAA, MOF was a significant risk factor. During the earlier, more critical period (<2 days), preoperative shock and postoperative inotropic support were especially a significant risk factor. During the recovery period (<30 days), intraoperative aortic clamping site and postoperative RRT were significant risk factors. We think that postoperative inotropic support, postoperative RRT, and postoperative MOF are mostly due to ischemic changes and coagulopathy caused by systemic and renal hypoperfusion due to hypovolemic shock, based on the massive hemorrhaging seen in RAAA patients, and suprarenal aortic clamping can also lead to renal hypoperfusion and ischemia. In the literature,2,3,9,13-18 preoperative factors were the ones most frequently suggested as risk factors of the postoperative mortality of RAAA. However, our report outlines significant relationships among preoperative, intraoperative, and postoperative factors in the postoperative mortality of RAAA.
Based on the risk factors mentioned above, there have been many efforts to decrease the postoperative mortality of RAAA.2-4,6,14,20-22 Kim, et al.,2 Scarcello, et al.4 and Beah, et al.14 asserted that operations should be performed as soon as possible before preoperative cardiac arrest develops. Cho, et al.3 reported the importance of a prompt diagnosis and recovery from a shock state by fluid resuscitation and transfusion, and they suggested that the rapid infusion system could be useful in this situation. Spinella, et al.20 and Gonzalez, et al.21 asserted the usefulness of "early plasma transfusion" or "hemostatic resuscitation" in massively hemorrhaging patients with equal ratios of fresh frozen plasma (FFP) to PRBC, and they suggested early plasma transfusion as a volume expander as well as a primary means for preventing coagulopathy. Mell, et al.6 demonstrated a marked decrease in mortality (15%) in RAAA patients who received equivalent transfusions of FFP : PRBC (high FFP), and the mortality of the group transfused at ratios similar to the traditional resuscitation ratios of 1 unit FFP : 3-4 units PRBC (low FFP) was markedly higher (39%), which was similar to the mortality rates of many published RAAA series. They also suggested that early plasma transfusion might correct coagulopathies from massive hemorrhages, resulting in greater oncotic pressure in the immediately postoperative period and a decrease in hypoperfusion, which, therefore, could decrease mortality.6 As another theory, permissive hypotension is a method used to decrease the risk of uncontrolled hemorrhaging.22 This method is a technique in which enough volume is given to maintain a systolic blood pressure of 80-100 mm Hg until aortic control is achieved.22 The goal of this technique is to prevent an increase in blood pressure from excessive volume administration in order to avoid uncontrolled hemorrhaging by converting a contained retroperitoneal hemorrhage into a free intraperitoneal hemorrhage.6 In these methods, the authors made an effort to perform prompt diagnosis and recovery from a shock state by fluid resuscitation and transfusion with high FFP. We mostly did not perform the permissive hypotension; however, we had several experiences with deaths that were thought to be due to the conversion of a contained retroperitoneal hemorrhage into a free intraperitoneal hemorrhage due to excessive volume administration. Therefore, we feel that we need to perform the permissive hypotension technique in the future.
Additionally, different operation techniques have been reported.23-27 Chang, et al.23 suggested the retroperitoneal approach for a postoperatively early recovery. The first series describing endovascular RAAA repair (REVAR) by Ohki and Veith24 from the Montefiore group included 12 patients treated with a custom-made stent graft, with a mortality rate of only 12%, suggesting that REVAR could potentially improve the traditionally high mortality rates associated with open repair. Since then, the dissemination of endovascular technology has allowed centers to offer EVAR to a wide range of patients with more complex anatomy, and to perform EVAR as the first line of treatment for RAAA in many cases.25 Mehta, et al.26 reported that they achieved a mortality rate of 18% with emergent endovascular repair of hemodynamically stable and unstable patients. Davenport, et al.27 asserted that the performance of EVAR in RAAA patients with favorable anatomy could potentially result in lower morbidity and decreased transfusion requirements, as compared with open repair. However, the present authors have not yet introduced EVAR for RAAA.
In conclusion, minimizing preoperative shock, intraoperative suprarenal aortic clamping, postoperative inotropic support, postoperative RRT, and postoperative MOF is imperative to minimizing the postoperative mortality of RAAA, according to our study. To do so, prompt diagnosis, operation, and securing proper blood flow to minimize massive hemorrhages and coagulopathies are most important. So, in the preoperative period, prompt diagnosis, quick recovery from a shock state by fluid resuscitation and transfusion with high FFP, and permissive hypotension to maintain the hemodynamic status prior to surgery need to be performed. In the intraoperative period, operative control of bleeding, maintenance of systemic perfusion, and infrarenal aortic clamping in infrarenal RAAA, regardless of neck length, as a possible means to avoid additional renal ischemic changes are vital. Of course, suprarenal aortic clamping in juxtarenal or suprarenal RAAA is obligatory. In the postoperative period, critical and proper care for maintenance of systemic perfusion and recovery of organ function must be undertaken.
Figures and Tables
Table 1
Univariate Analysis of Preoperative Risk Factors Associated with Postoperative Mortality of RAAA
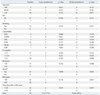
n, number; yr, year; M, male; F, female; IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; CRF, chronic renal failure; Cr, creatinine; Hb, hemoglobin; PR, pulse rate; ER, emergency room; OR, operation room; RAAA, ruptured abdominal aortic aneurysm.
*Circulatory Shock, systolic blood pressure <80 mm Hg at hospitalization (before procedure).10
Table 2
Univariate Analysis of Intraoperative Risk Factors Associated with Postoperative Mortality RAAA
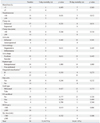
Table 3
Univariate Analysis of Postoperative Risk Factors Associated with Postoperative Mortality of RAAA
Notes
References
1. Hardman DT, Fisher CM, Patel MI, Neale M, Chambers J, Lane R, et al. Ruptured abdominal aortic aneurysms: who should be offered surgery? J Vasc Surg. 1996. 23:123–129.

2. Kim IH, Kim DI, Huh SH, Lee SJ, Lee BB. Factors that affect the survival rate of ruptured abdominal aortic aneurysm. J Korean Soc Vasc Surg. 2001. 17:199–202.
3. Cho MJ, Yoon HJ, Park JY, Huh S, Kim YW. The risk factors influencing postoperative mortality in the patients with ruptured abdominal aortic aneurysm. J Korean Soc Vasc Surg. 2004. 20:208–213.
4. Scarcello E, Ferrari M, Rossi G, Berchiolli R, Adami D, Romagnani F, et al. A new preoperative predictor of outcome in ruptured abdominal aortic aneurysms: the time before shock (TBS). Ann Vasc Surg. 2010. 24:315–320.

5. Noel AA, Gloviczki P, Cherry KJ Jr, Bower TC, Panneton JM, Mozes GI, et al. Ruptured abdominal aortic aneurysms: the excessive mortality rate of conventional repair. J Vasc Surg. 2001. 34:41–46.

6. Mell MW, O'Neil AS, Callcut RA, Acher CW, Hoch JR, Tefera G, et al. Effect of early plasma transfusion on mortality in patients with ruptured abdominal aortic aneurysm. Surgery. 2010. 148:955–962.

7. Lee RW, Rhodes JM, Singh MJ, Davies MG, Wolford HY, Diachun C, et al. Is there a selection bias in applying endovascular aneurysm repair for rupture? Ann Vasc Surg. 2008. 22:215–220.

8. Davies RS, Dawlatly S, Clarkson JR, Bradbury AW, Adam DJ. Outcome in patients requiring renal replacement therapy after open surgical repair for ruptured abdominal aortic aneurysm. Vasc Endovascular Surg. 2010. 44:170–173.

9. Halpern VJ, Kline RG, D'Angelo AJ, Cohen JR. Factors that affect the survival rate of patients with ruptured abdominal aortic aneurysms. J Vasc Surg. 1997. 26:939–945.

10. Bonardelli S, Cervi E, Maffeis R, Nodari F, De Lucia M, Guadrini C, et al. Open surgery in endovascular aneurysm repair era: simplified classification in two risk groups owing to factors affecting mortality in 137 ruptured abdominal aortic aneurysms (RAAAs). Updates Surg. 2011. 63:39–44.

11. Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Subcommittee on Reporting Standards for Arterial Aneurysms. Ad Hoc Committee on Reporting Standards. Society for Vascular Surgery and North American Chapter. International Society for Cardiovascular Surgery. Suggested standards for reporting on arterial aneurysms. J Vasc Surg. 1991. 13:452–458.

12. Fitzgerald JF, Stillman RM, Powers JC. A suggested classification and reappraisal of mortality statistics for ruptured atherosclerotic infrarenal aortic aneurysms. Surg Gynecol Obstet. 1978. 146:344–346.
13. Hsiang YN, Turnbull RG, Nicholls SC, McCullough K, Chen JC, Lokanathan R, et al. Predicting death from ruptured abdominal aortic aneurysms. Am J Surg. 2001. 181:30–35.

14. Beah MJ, Kwon TW, Cho YP, Kim HS, Kim GE. Factors affecting mortality rate of ruptured abdominal aortic aneurysm. J Korean Soc Vasc Surg. 2000. 16:33–37.
15. Rutledge R, Oller DW, Meyer AA, Johnson GJ Jr. A statewide, population-based time-series analysis of the outcome of ruptured abdominal aortic aneurysm. Ann Surg. 1996. 223:492–502.

16. Evans SM, Adam DJ, Bradbury AW. The influence of gender on outcome after ruptured abdominal aortic aneurysm. J Vasc Surg. 2000. 32:258–262.

17. Boyle JR, Gibbs PJ, King D, Shearman CP, Raptis S, Phillips MJ. Predicting outcome in ruptured abdominal aortic aneurysm: a prospective study of 100 consecutive cases. Eur J Vasc Endovasc Surg. 2003. 26:607–611.

18. Alric P, Ryckwaert F, Picot MC, Branchereau P, Colson P, Mary H, et al. Ruptured aneurysm of the infrarenal abdominal aorta: impact of age and postoperative complications on mortality. Ann Vasc Surg. 2003. 17:277–283.

19. Sinicrope RA, Serra RM, Engle JE, Nicholas GG, Devine MF, Schoolwerth AC. Mortality of acute renal failure after rupture of abdominal aortic aneurysms. Am J Surg. 1981. 141:240–242.

20. Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008. 64:2 Suppl. S69–S77.

21. Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007. 62:112–119.

22. Roberts K, Revell M, Youssef H, Bradbury AW, Adam DJ. Hypotensive resuscitation in patients with ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2006. 31:339–344.

23. Chang BB, Shah DM, Paty PS, Kaufman JL, Leather RP. Can the retroperitoneal approach be used for ruptured abdominal aortic aneurysms? J Vasc Surg. 1990. 11:326–330.

24. Ohki T, Veith FJ. Standard and new treatments for abdominal aortic aneurysms: the value of the Montefiore endovascular grafts for difficult aneurysms. Jpn Circ J. 1999. 63:829–837.

25. Ricotta JJ 2nd, Malgor RD, Oderich GS. Ruptured endovascular abdominal aortic aneurysm repair: part II. Ann Vasc Surg. 2010. 24:269–277.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download