Abstract
Purpose
To investigate the causes of varicocele recurrence and assess the use of embolization and subinguinal varicocelectomy in its treatment in patients with angiography and subinguinal varicocelectomy.
Materials and Methods
The present study involved 15 patients with recurrent varicoceles. The mean patient age was 21.2 years (range: 12-42 years). Preoperative angiography was performed in 11 patients. Embolization was used in patients with patent internal spermatic veins (ISVs). Patients without patent ISVs or preoperative angiography underwent magnification-assisted subinguinal varicocelectomy which included testicular retrieval and ligation of all collateral veins except arteries and deferential veins.
Results
Seven among 11 patients (64%) which had preoperative angiography had patent ISVs and underwent embolization and 8 patients underwent subinguinal varicocelectomy. Of those 8 patients, 6 had dilated ISVs and external spermatic veins (ESVs), one had dilated ISVs and gubernacular veins, and one had dilated ISVs, ESVs and gubernacular veins. No patient experienced recurrence or testis atrophy.
Conclusion
Patent ISVs or collateral veins may be the cause of recurrence after varicocelectomy. Angiographic embolization was successful in 64% of recurrent varicoceles patients with patent ISVs. However, microscope-assisted subinguinal varicocelectomy may be the best overall treatment for patients with recurrent varicoceles.
The pathophysiology of adolescent varicocele may be multifactorial. Traditionally, varicocele formation has been attributed to one of three primary factors: increased venous pressure in the left renal vein, collateral venous anastomoses, and incompetent valves of the internal spermatic vein (ISV).1 A possible cause of varicoceles is reflux in the collateral veins including the cremasteric and external pudendal veins or gubernacular veins, all of which drain into the iliac vein.
Microsurgical subinguinal varicocelectomy with delivery of the testis provides direct visual access to all avenues of testicular venous drainage and is reported to result in a significant decrease in the incidence of varicocele recurrence.2,3 Many urologists believe that collateral reflux that may be a factor in recurrence.4,5 However, Franco reported that cremasteric vein dilatation is not due to reflux but probably venous overflow, and that surgical strategies aimed at ligation of collateral veins are inadequate to reduce varicocele recurrence.6 Franco also stated that retrograde and antegrade venographic findings indicate that varicocele is a disease of the ISV only.7
The causes of varicocele recurrence remain a matter of conjecture. Such conjecture may reflect the fact that previous studies used only a single approach such as radiologic assess including scrotal color Doppler ultrasounds (SCDU) and angiography or surgical assess including intraoperative anatomy and venography to evaluate varicocele recurrence. The present study investigated varicocele recurrence in 15 patents. We evaluated the causes of varicocele recurrence by using the findings from preoperative angiographic studies and subinguinal varicocelectomies, and assessed the use of embolization and subinguinal varicocelectomy with delivery of the testis as treatments.
Between May 2005 and Dec 2008, 159 patients with grade 3 varicocele underwent inguinal varicocelectomy (n=75), laparoscopic varicocelectomy (n=80) or embolization (n=4) treatment. Indications for initial varicocelectomy were abnormal semen results, scrotal discomfort or pain, visible varicocele (grade 3), testicular hypotrophy, bilaterality, and the patient's request or anxiety due to his condition. A diagnosis of varicocele was based on a physical examination in the upright and supine positions using Valsalva's maneuver. Varicoceles were graded according to Dubin and Amelar.8 Eleven (6.9%) of those 159 patients experienced recurrence. To detect varicocele recurrence, we used physical examination and SCDU. The present study comprised those 11 patients plus another 4 patients referred to us from other institutions, to make a study population of 15 patients. Clinical data were obtained by retrospective review of the medical records of all 15 patients. The mean patient age was 21.2 years (range: 12-42 years). Median follow-up was 23 months. None of the patients except one was married and all patients had left-sided recurrence. Five patients had grade 2 varicocele and 10 patients had grade 3 varicocele. Preoperatively, patients underwent assessment using SCDU (HDI 5000; Philips, Bothell, WA, USA). Angiography (Integris 3000, Philips, Best, the Netherlands) was performed in 11 patients. We attempted to perform angiography in all of our 15 patients, however, we could not perform angiography in 4 as 3 patients refused the procedure and one had the contrast allergies. All images were reviewed on a Picture Archiving and Communications System workstation monitor (m-view, Marotech, Seoul, Korea).
Angiography was performed by an experienced interventional radiologist. Patients with persistent and communicated ISVs according to angiography were treated by embolization. Embolization was performed by placing 3% sodium tetradecyl sulfate foam and/or 0.035 Ternado coils (Cook, Bloomington, IN, USA) into the patent ISVs. The gonadal vein was selectively catheterized by using right femoral venous access with 4 Fr cobra catheter.9
Patients without patent veins or preoperative angiography underwent an open subinguinal varicocelectomy with delivery of the testis using magnification. For this procedure, spinal anesthesia was used, and patients were placed in the supine position. When the spermatic cord was reached, the external spermatic, cremasteric and internal spermatic fascia were opened in the longitudinal direction. Dilated external spermatic veins (ESVs) and ISVs were identified using 2.5× loupe or 8× operating microscope magnification. Spermatic cord dissection was continued, and the testicular artery and lymphatics were preserved. The vas deferens and deferential vessels, cremasteric muscle, and a majority of the lymphatics and arteries were preserved as much as possible. Using delivery of the testis, gubernacular veins or transscrotal collaterals were ligated. Before the procedures ended, the patients were changed to a slight head-up position and ipsilateral testicles were squeezed to identify the remaining varicose veins. The wound and scrotal contents were examined routinely at 3, 6, 12, and 24 months, and also whenever requested by the patient. Median follow-up for the 15 patients was 23 months.
The mean varicocele recurrence time for the 15 patients was 5.3 months (range: 0.75-13 months) after the initial treatment.
Angiography showed that no patient had evidence of reflux of contrast from the left iliac vein into the left pampiniform plexus. Seven among 11 patients (64%) which had preoperative angiography had patent ISVs and underwent embolization. Three patients had no patent ISVs (Fig. 1) and one patient failed to demonstrate gonadal vein in renal vein (Fig. 2). Eight patients without patent ISVs or preoperative angiography underwent subinguinal varicocelectomy. Six of those 8 patients had dilated ISVs and ESVs (Fig. 3), one had dilated ISVs and gubernacular veins, and one had dilated ISVs, ESVs and gubernacular veins (Table 1). One patient had hydrocele after initial treatment of varicocele and this was also treated in one session during re-do surgery.
The preoperative and postoperative sperm counts in men with re-do surgery were 1.95×106 and 2.38×106 per mL, respectively. Our current study had only a small number of patients and there was no statistically significant difference. Postoperatively, all 8 patients who underwent subinguinal varicocelectomy experienced scrotal inflammation that lasted for 4-21 days, and the condition healed naturally over time. No patient experienced another recurrence or testis atrophy.
Varicocele treatments include macroscopic inguinal or subinguinal varicocelectomy, angiographic embolization, microscopic inguinal or subinguinal varicocelectomy, and laparoscopic varicocelectomy.10,11 Surgery is currently the most popular treatment for varicocele patients with signs of abnormal semen, testicular hypotrophy or pain. The recurrences rates following varicocele repair range from 0.6-35% depending upon the technique used.3,10 Although previous two studies have compared various varicocele treatment approaches, the optimum treatment remains a matter of debate.
The pathophysiology of varicocele has been attributed to one of three primary factors: increased venous pressure in the left renal vein or gonadal vein, reflux in the collateral veins, and incompetent ISV valves.1 The processes underlying recurrence appear to be similar.
The most common cause of persistent or recurrent varicocele after surgical repair involves the ISVs.12,13 Redundancies of the gonadal veins confined to the region in or near the inguinal canal appear to be responsible for the majority of post-surgical persistent or recurrent varicocele.14 Macroscopic inguinal or subinguianl varicocelectomy performed without optical magnification may miss smaller internal spermatic veins that may later dilate and cause recurrence.10 The use of microscope magnification allows to identify the testicular artery, lymphatics, and small venous channels, which assists in the preservation of arterial and lymphatic vessels, and also allows to completely ligate the spermatic veins, which in turn minimizes the risk of postoperative complications. These measures significantly decrease the incidence of hydrocele formation, testicular artery injury, and varicocele recurrence.15 This cause of recurrence was demonstrated in the previous reports: inguinal and retroperitoneal collateral venous channels of ISV were a major etiology in varicocele ligation failure.16,17
The second most important factor influencing varicocele recurrence is collateral venous anastomosis. Franco concluded that cremasteric vein dilation was probably due to venous overflow and that surgical strategies aimed at ligation of the collateral veins were ineffective for reducing varicocele recurrence.6 In addition, he surmised that varicocele was a disease of the ISV only.7 In contrast, using venography of the gonadal, renal and common iliac vein, Coolsaet concluded that varicoceles were due to reflux into the ISV (67% of cases), the extrafunicular veins (20% of cases) or both veins (14%).18 Many urologists believe that collateral reflux may be a factor in recurrence.4,5 Microsurgical inguinal varicocelectomy with delivery of the testis provides direct visual access to all avenues of testicular venous drainage and is reported to result in a significant decrease in the incidence of varicocele recurrence.2,3
The present study may support the significant role of extrafunicular veins in varicocele recurrence. We think that the persistent ISVs and collateral veins of ISV are the causes of varicocele recurrence. However, we are at a loss to explain the exact mechanism of varicocele recurrence and which one between persistent ISVs and collaterals influences the recurrence more likely. Our angiography studies could not identify reflux from the iliac vein into the pampiniform plexus in any patient, and showed that 7 patients had persistent ISVs. Although angiography showed that 4 patients failed to demonstrate patent ISVs, dilated ISVs were observed in those patients during surgery. Those patients had also dilated collateral veins including ESVs or gubernacular veins. We are not certain whether these dilated ISVs were caused by collateral veins or not. As Franco pointed out, we failed to find remnant ISV channel because of lack of angiographic skill or anatomic knowledge. Bähren, et al.19 and Murray, et al.12 evaluated ISV varicocele using venography, and each study created five classifications. However, we were unable to classify the present patent ISVs based on those classifications. It is quite possible that ISV dilation was caused by reflux via ESV or other collateral veins.
In the current study, we used subinguinal varicocelectomy with loupe or microscope magnification, which enabled us to ligate dilated collateral veins and small venous channels other than the testicular artery and lymphatics. The subinguinal varicocelectomy allows for testicular delivery, enabling identification and ligation of collateral veins such as the external spermatic (cremasteric), external pudendal, and gubernacular veins, thus minimizing the risk of recurrence. Libman, et al.20 reported that the number of arteries and lymphatic channels identified and preserved at a redo subinguinal microsurgical varicocelectomy was comparable to that observed during a primary microsurgical varicocelectomy. There were no further recurrences after redo varicocelectomy in the current study. Although many urologists recommend embolization following a failed surgical varicocelectomy, the method has 5.8-20% recurrence rates.14,21-23 In contrast, however, subinguinal microscopic repair of recurrent varicoceles has a recurrence rate of 2%.21 Angiographic embolization may be appropriate in a limited number of recurrent varicoceles patients with patent ISVs. However, angiographic embolization is a minimally invasive outpatient procedure and has many advantages such as no need for general anesthesia, early recovery, and decreased morbidity (nearly zero percent) such as formation of a hydrocele, testicular atrophy, and epididymorchitis.24,25
Microscope-assisted subinguinal varicocelectomy may be the best overall treatment for patients with recurrent varicoceles. Of course, larger number of patients and prospective studies are needed to more clearly define the results and conclusions regarding the causes or best treatments of recurrent varicoceles.
Figures and Tables
Fig. 1
Angiographic findings from a varicocele recurrence patient. Note that there is no patent internal spermatic vein and no reflux of contrast from the left iliac vein into the left pampiniform plexus.
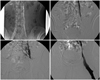
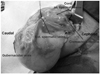
Fig. 3
Surgical findings. Cremasteric and internal spermatic fascia were opened in the longitudinal direction. Note that the dilated internal and external spermatic veins (cremasteric veins) are clearly identifiable.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Jae Wook Kim for his warming supports regarding radiologic work. I and the other authors have no possible conflicts of interest, sources of financial support, corporate involvement, patent holdings, etc.
References
1. Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001. 7:473–481.
2. Beck EM, Schlegel PN, Goldstein M. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 1992. 148:1190–1194.

3. Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992. 148:1808–1811.

4. Lee JW, Paick JS, Kim SW. Microsurgical subinguinal varicocelectomy: comparison of pediatric and adult patients. Korean J Urol. 2008. 49:1029–1034.

5. Al-Kandari AM, Shabaan H, Ibrahim HM, Elshebiny YH, Shokeir AA. Comparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trial. Urology. 2007. 69:417–420.

6. Franco G, Iori F, de Dominicis C, Dal Forno S, Mander A, Laurenti C. Challenging the role of cremasteric reflux in the pathogenesis of varicocele using a new venographic approach. J Urol. 1999. 161:117–121.

7. Franco G, Leonardo C. Is selective internal spermatic venography necessary in detecting recurrent varicocele after surgical repair? Eur Urol. 2002. 42:192–193.

8. Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970. 21:606–609.

9. Lord DJ, Burrows PE. Pediatric varicocele embolization. Tech Vasc Interv Radiol. 2003. 6:169–175.

10. Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009. 30:33–40.

11. Al-Said S, Al-Naimi A, Al-Ansari A, Younis N, Shamsodini A, A-sadiq K, et al. Varicocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approaches. J Urol. 2008. 180:266–270.

12. Murray RR Jr, Mitchell SE, Kadir S, Kaufman SL, Chang R, Kinnison ML, et al. Comparison of recurrent varicocele anatomy following surgery and percutaneous balloon occlusion. J Urol. 1986. 135:286–289.

13. Kaufman SL, Kadir S, Barth KH, Smyth JW, Walsh PC, White RI Jr. Mechanisms of recurrent varicocele after balloon occlusion or surgical ligation of the internal spermatic vein. Radiology. 1983. 147:435–440.

14. Sze DY, Kao JS, Frisoli JK, McCallum SW, Kennedy WA 2nd, Razavi MK. Persistent and recurrent postsurgical varicoceles: venographic anatomy and treatment with N-butyl cyanoacrylate embolization. J Vasc Interv Radiol. 2008. 19:539–545.

15. Abdel-Maguid AF, Othman I. Microsurgical and nonmagnified subinguinal varicocelectomy for infertile men: a comparative study. Fertil Steril. 2010. 94:2600–2603.

16. Rais-Bahrami S, Montag S, George AK, Rastinehad AR, Palmer LS, Siegel DN. Angiographic Findings of Primary Versus Salvage Varicoceles Treated with Selective Gonadal Vein Embolization: An Explanation for Surgical Treatment Failure. J Endourol. 2012. [Epub ahead of print].

17. Feneley MR, Pal MK, Nockler IB, Hendry WF. Retrograde embolization and causes of failure in the primary treatment of varicocele. Br J Urol. 1997. 80:642–646.

18. Coolsaet BL. The varicocele syndrome: venography determining the optimal level for surgical management. J Urol. 1980. 124:833–839.

19. Bähren W, Lenz M, Porst H, Wierschin W. Side effects, complications and contraindications for percutaneous sclerotherapy of the internal spermatic vein in the treatment of idiopathic varicocele. Rofo. 1983. 138:172–179.

20. Libman JL, Segal R, Baazeem A, Boman J, Zini A. Microanatomy of the left and right spermatic cords at subinguinal microsurgical varicocelectomy: comparative study of primary and redo repairs. Urology. 2010. 75:1324–1327.

21. Grober ED, Chan PT, Zini A, Goldstein M. Microsurgical treatment of persistent or recurrent varicocele. Fertil Steril. 2004. 82:718–722.

22. Punekar SV, Prem AR, Ridhorkar VR, Deshmukh HL, Kelkar AR. Post-surgical recurrent varicocele: efficacy of internal spermatic venography and steel-coil embolization. Br J Urol. 1996. 77:124–128.

23. Kim J, Shin JH, Yoon HK, Ko GY, Gwon DI, Kim EY, et al. Persistent or recurrent varicocoele after failed varicocoelectomy: outcome in patients treated using percutaneous transcatheter embolization. Clin Radiol. 2012. 67:359–365.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download