Abstract
Purpose
The nephrotic syndrome (NS) is characterized by the favorable response to glucocorticoid therapy and the development of NS may be associated with dysfunctional immune systems. In order to investigate the serum immunoglobulin E (IgE) levels and cytokines activity in pediatric NS, the total of 32 steroid responsive NS patients and 5 healthy controls were enrolled in this study.
Materials and Methods
All patients were divided into two groups according to the initial serum IgE levels, such as normal and high IgE group, and their clinical characteristics were evaluated. In addition, serum levels of interleukin (IL)-4, IL-5, IL-10 and transforming growth factor (TGF)-β were compared and correlated with serum albumin, proteinuria by means of disease severity, and cytokines.
Results
In the high IgE group, the higher comorbidity of allergic diseases and relapsing rate, the longer duration of steroid therapy before initial remission, and the higher serum IL-4 and IL-5 levels were found. In all patients, initially higher serum levels of IL-4 and IL-5 declined to normal levels after steroid therapy, whereas the serum IL-10 levels showed no significant difference between nephrotic phase (heavy proteinuria) and remission phase (no proteinuria) of NS. The serum TGF-β levels of the nephrotic phase were significantly lower than those of remission phase or control group, and returned to normal control levels after steroid therapy.
Idiopathic nephrotic syndrome (NS) is characterized by generalized edema, heavy proteinuria, hypoalbuminemia, and hyperlipidemia, and minimal change disease (MCD) is the most common form of idiopathic NS, accounting for 90% of patients under the age of 10 years and more than 50% of older children.1,2
Although the etiology of idiopathic NS is unknown, several candidate factors have been identified. After the first report of hypersensitivity and NS by Hardwicke3 in 1959, numerous studies have reported an association between NS and allergy.4,5 Several studies have demonstrated elevated serum immunoglobulin E (IgE) levels in NS patients and hypothesized its active role in the pathophysiology, however, a direct relationship between IgE and the pathogenesis of NS is controversial.6,7
Meanwhile, it was proposed that abnormal T cell response and Th2 cytokines are strongly related to the pathogenesis of NS.6,8 Various evidences indicate that lymphocytes derived from an abnormal immune system alter the permeability of the glomerular capillary wall, suggesting that immune dysfunction plays a key role in the pathogenesis of NS.8-11 Activated macrophages and Th2 lymphocytes may be involved in the pathogenesis of NS, and T-cell dysfunction leads to changes in cytokines, causing a loss of negatively charged glycoproteins within the glomerular capillary wall.12,13
Recently, regulatory T cells (Treg) and T-helper17/Treg imbalance has also been suggested as a potential factor in the pathogenesis of MCD and Treg dysfunction and decrease of their related cytokines, transforming growth factor (TGF)-β and interleukin (IL)-10, have been reported.14,15 Especially, TGF-β was suggested as a protective cytokine.14
In this study, we evaluated the clinical characteristics of pediatric steroid-responsive NS patients according to the initial serum IgE levels, and several immune mediators such as Th2 cytokines (IL-4, IL-5) and Treg related cytokines (TGF-β, IL-10) were compared according to their remission status.
The study protocol was conducted after approval by the Institutional Review Board of Chungnam National University Hospital, Daejeon, Korea and was carried out after informed consent was obtained from the patients or their legitimate guardians.
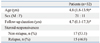
From September, 2004 to July, 2010, the total of 32 patients (21 boys and 11 girls, aged 1.8 to 13.9 years) who underwent prednisolone therapy (17 steroid-sensitive and 15 steroid-dependent) for idiopathic nephrotic syndrome at the Department of Pediatrics, Chungnam National University Hospital were enrolled in this study (Table 1). The median duration of follow-up was 4.7 years. The patients with congenital or secondary renal diseases were excluded. The diagnostic criteria for idiopathic NS were based on the International Study of Kidney Disease in Children.16 Five healthy children (1 boy and 4 girls, aged 6 to 8 years) were included as normal control group.
The children during their first episode of NS were admitted to the pediatric wards prior to steroid treatment, and clinical data including age, gender, and blood pressure were collected. The blood sample was drawn to evaluate white blood cell count, eosinophil count, platelet, protein, albumin, cholesterol, triglyceride, blood urea nitrogen, creatinine, electrolytes, and various immune mediators, such as complement 3 and 4, antineutrophil antibody, antistreptolysin O and C-reactive protein.
The proteinuria, hematuria and renal functions were screened by spot and 24-hour urine analysis. When MCD was presumed, prednisolone therapy was initiated at a dose of 60 mg/m2/day after confirming a negative purified protein derivative skin test.
All of the steroid responsive NS patients were divided into a normal IgE group and a high IgE group according to the initial serum IgE levels. The patients who showed higher IgE levels, measuring more than upper 2 standard deviation score of the age-adjusted reference range, were defined as high IgE group.17 Remission was characterized by a marked reduction in proteinuria (to <4 mg/m2/hr or urine albumin dipstick of 0 to trace for 3 consecutive days) in association with resolution of edema and normalization of serum albumin to at least 3.5 g/dL. Relapse was defined as recurrence of massive proteinuria (>40 mg/m2/hr, urine protein/creatinine ratio >2.0 mg/mg, or urine albumin dipstick ≥2+ on 3 consecutive days), most often in association with recurrence of edema.2
During the nephrotic phase prior to steroid treatment and remission phase, the serum was separated from whole blood and was kept at -70℃ for immune cytokine assay. The serum IgE levels were analyzed by Hitachi optigen allergen specific IgE assay system Korean inhaler panel (Hitachi chemical diagnostic, Inc., Mountain View, CA, USA) based on manufacturer's method. The serum levels of IgG, IgA, and IgM were determined by standard enzyme immunoassay techniques (Seiken, Denka Seika, Nigata-ken, Japan).
The serum IL-4, IL-5 and TGF-β levels were measured by human platinum enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA, USA). The serum IL-10 levels were detected by human high sensitivity ELISA kit (eBioscience). Briefly, standard dilutions and samples were pipetted into the wells. And the concentrated streptavidin-horseradish peroxidase solution was added to all the wells. After a wash to remove any unbound reagent, a TMB substrate solution was added to the wells and the intensity of the color was measured by a spectrophotometer at 450 µm.
The clinical and laboratory information of the NS patients were expressed as a median and range (minimum to maximum) by frequent analysis. The configuration with NS patients between normal IgE and high IgE levels were analyzed by using Mann-Whitney U test and Fisher's exact test. The Kruskall-Wallis test and Scheffe's post hoc comparison were used to compare the cytokines between NS patients with nephrotic and remission phase and healthy controls.
Significant correlation between circulating cytokines and serum albumin levels was assessed by linear regression analysis. The data were analyzed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA), and a p value<0.05 was considered significant.
Subjects were divided into two groups; 12 patients (7 boys and 5 girls) who had normal IgE levels and 20 patients (14 boys and 6 girls) who had high IgE levels. The clinical characteristics among the groups were compared by Mann-Whitney U test and Fisher's exact test. At the time of initial diagnosis, the data of their age, gender, and the laboratory findings of blood chemistry such as white blood cell count, eosinophil count, serum protein, albumin, cholesterol, IgG, IgA, IgM, immune mediators, and 24-hour urine protein/creatinine did not show any significant differences between the two groups (Table 2). However, the duration of clinical remission was significantly shorter in the normal IgE group than in the high IgE group (p=0.005). The relapse rate (p=0.003) and the concomitant allergic diseases (p<0.001) were more frequent in the high IgE group. The median (range) time to the initial remission was 8.5 (7.0-12.0) days in the normal IgE group and 12.5 (8.0-32.0) days in the high IgE group (p=0.005) and the median (range) duration of total steroid therapy was 4.0 (2.5-22.0) months in the normal IgE group and 54.0 (3.0-108.0) months in the high IgE group (p=0.006) (Table 2). Total follow-up durations of two groups were same.
In the normal IgE group, the median (range) initial serum levels of each IL-4 and IL-5 were 1.3 (1.0-1.4) and 1.3 (0.9-1.4) pg/mL, respectively. In the high IgE group, the serum levels of IL-4 and IL-5 was 1.9 (1.0-6.2) and 2.0 (1.1-4.4) pg/mL, respectively. Initial serum levels of IL-4 (p=0.018) and IL-5 (p=0.039) were significantly higher in the high IgE group than in the normal IgE group (Fig. 1). However, the serum levels of IL-10 and TGF-β showed no difference between them (data are not shown).
There were no changes in the median (range) levels of serum IgE according to the treatment state (nephrotic or remission phase) between the normal and high IgE groups (Fig. 2).
To explore the disease activities and the roles of cytokines, the serum levels of IL-4, IL-5, IL-10, and TGF-β were examined in both NS patients and healthy controls. The serum levels of IL-4 were significantly higher in the nephrotic phase of NS than in the remission phase (p<0.001) and control group (p<0.001). However no difference was found between remission phase of NS and control group (p=0.991) (Fig. 3A).
The serum levels of IL-5 were higher also in the nephrotic phase patient than those of remission phase of NS (p=0.051) and controls (p=0.028), but there were no significant differences between the patients with remission phase and control group (p=0.858) (Fig. 3B). The serum levels of IL-10 did not show any differences between both groups (Fig. 3C).
The serum levels of TGF-β in the nephrotic phase of NS patients were significantly lower than those of remission phase (p<0.001) and control group (p<0.001). However, there was no statistically significant difference between patients with remission phase and control group (p=0.576) (Fig. 3D).
Linear regression analysis showed that the serum levels of IL-4 had a negative correlation with that of albumin (R2=0.259, p=0.004) (Fig. 4A), while the serum levels of IL-5 and IL-10 did not show any correlations with that of albumin (R2=0.170, p=0.113 and R2=0.001, p=0.571, respectively) (Fig. 4B and C). On the other hand, the serum level of TGF-β had a positive correlation with that of albumin (R2=0.501, p<0.001) (Fig. 4D).
The pathogenesis of idiopathic NS is not thoroughly understood. However, the hypothesis was put forward in 1974 that idiopathic NS is an immune disorder with increased levels of lymphocyte-derived permeability factors. Since then, numerous lines of evidence have been presented, suggesting that immune mediators play a pivotal role in its pathogenesis.6-8,10,18
Although a pathogenic relationship remains to be confirmed, the serum levels of IgE show a close relationship to NS, and many studies have shown a strong association between idiopathic NS and atopic disorders.4,5 IgE levels were significantly higher in NS patients with atopy than in nonatopic patients, especially during the relapse phase of NS compared with the remission phase, implicating IgE as a barometer of disease severity and giving it prognostic value.6,19 In our present study, in a comparison of the clinical characteristics of NS patients with normal IgE and those with high IgE, the high-IgE group required a significantly longer time to reach their initial remission, was more susceptible to frequent relapse and comorbid allergic diseases, and required longer steroid therapy to recover from the nephrotic phase than the normal-IgE group. Serum IgG levels were lower in NS patients, whereas the levels were not different between the normal- and high-IgE groups. It is not certain whether the increased levels of IgE in idiopathic NS are pathogenic or co-incident, nevertheless, our results suggest that the regulation of total IgE production correlates with the disease activity and outcome of NS.20 However, it should be noted that part of the nephrotic children had persistently normal serum IgE levels, indicating different etiologies in the pathogenesis of NS.
The signals for IgE synthesis are delivered by IL-4 or IL-13. In relapsed NS, activated T cells increase the secretion of IL-4 and IL-13, which could induce isotype switching in IgE. IL-5 also promotes the production of IgE, whereas IFN-γ inhibits the secretion of IgE.13,21,22 In our study, high-IgE group displayed significantly elevated serum IL-4 and IL-5 levels. Thus, we suggest that the increased production of IL-4 and IL-5 in idiopathic NS may indicate that these cytokines are involved in the enhanced production of serum IgE. Furthermore, allergic treatment alone could not induce the remission of NS, suggesting that the elevated serum IgE in some patients can not cause their NS, but rather reflects allergic responses to the perturbation of their humoral immunity.4,23
In order to evaluate immunologic pattern and their modulating serum cytokines and to analyze the correlation with disease activity in children with idiopathic NS, we evaluated initial serum levels of IL-4, IL-5, IL-10 and TGF-β and their kinetics during treatment with or without relapse. A number of reports have shown that activated T cells of idiopathic NS are driven toward the Th2 phenotype.13
IL-4 is the most important Th2 cytokine which mediates IgE synthesis.24 In NS, IL-4 can act on glomerular visceral epithelial cells by binding IL-4 receptor, and plays a role in glomerular permeability.25 This suggests that, regardless of atopy, IL-4 may be one of the cytokines associated with the pathogenesis of proteinuria and also be a predictive factor for disease severity in NS. In our present study, the serum levels of IL-4 were higher in the nephritic phase of NS than the remission phase and control group and were correlated with serum albumin levels. However, this seems to be variance with the results in many previous studies that serum IL-4 levels decreased during relapse but increased in nephritic patients with long-term remission.26
Several authors have reported that serum IL-5 levels in the nephrotic phase were higher than in the remission phase and Ohtomo, et al.27 reported that suplatast tosilate, which suppresses IL-4 and IL-5 production, was effective for steroid reduction in idiopathic steroid sensitive NS.13,23 We also found that serum IL-5 levels were higher in the nephrotic phase than in the remission phase with steroid treatment. However, because other hither-to-uncharacterized factors might have been needed to induce idiopathic NS, further studies should be conducted to clarify the effects of IL-4 and IL-5 on idiopathic NS.
Recently, regulatory T cells (Treg cells) have been described as subsets distinct from Th1 and Th2 cells. Treg cells expressing the forkhead/winged helix transcription factor (Foxp3) have an anti-inflammatory role and maintain tolerance to self-components through direct contact with cells or by releasing anti-inflammatory cytokines such as IL-10 and TGF-β.28,29 Treg cell dysfunction has been found in several kidney disease animal models.30-32 A reduction in Treg cells induces local tissue inflammation, which may be related to the pathogenesis of proteinuria and the propagation of idiopathic NS.33 In relapsed idiopathic minimal NS, Treg cells have an impaired capacity to suppress T-effector-cell proliferation.14 Liu, et al.15 reported that Th17/Treg ratios were increased along with increased proteinuria and decreased albumin levels in adult patients with minimal change NS.
TGF-β has been shown to regulate both immunogenic and immunosuppressive response, depending on the cellular environment.34 Therefore, overall systemic effects of TGF-β must be understood within the context of other collaborative networks involving other immunoregulatory signals.
TGF-β is often associated with the progression of kidney disease, and exhibits fibrogenic and proinflammatory properties in the kidney.35-37 Proteinuria predominantly involves albumin, which triggers the positive feedback loop for TGF-β expression, subsequently inhibiting albumin endocytosis.38 Prolonged proteinuria gives rise to increased TGF-β, which modulates the expression of the extracellular matrix, leading to glomerulosclerosis and interstitial fibrosis, and retarding the progression to renal failure.39
On the other hand TGF-β may promote the expression of Foxp3 and induce the differentiation of Treg cells that which originate from CD4+CD25-T cells.40,41 TGF-β is the inhibitor of Th17 differentiation in humans,42 and it also inhibits the vascular permeability factor released by T cells in normal subjects and patients with minimal change disease.9 In the present study, we found that TGF-β and IL-10 concentrations decreased in minimal change NS patients and positively correlated with circulating Treg frequencies, which suggested that TGF-β and IL-10 may play a protective role, partly through inducing the production of Treg cells, and act as one of the effective factors of Treg cells.15
IL-10 is also pleiotropic cytokine that can exert either immunosuppressive or immunostimulatory effects on a variety of cell types and plays a central role in controlling inflammatory processes.43 Previous studies have shown that IL-10 is involved in the positive feedback loops with TGF-β and acts at local sites of inflammation, whereas TGF-β is involved in the systemic immune response related to humoral lymphoproliferation.44,45
In this study, the initial serum levels of lL-10 and TGF-β did not differ between the normal- and high-IgE groups, whereas the initial serum TGF-β levels in the nephrotic phase of NS were lower than in the remission phase or in the healthy controls, and differed significantly according to the degree of proteinuria and serum albumin level. However, serum lL-10 levels were not significantly different between the groups. The results of this study suggest that insufficient levels of TGF-β in the nephrotic phase of NS may aggravate the inflammatory responses and contribute to the induction of proteinuria. To better understand the nature, regulation, and function of Treg cells and their cytokines in idiopathic NS, further studies should be carried out.
Figures and Tables
Fig. 1
Comparisons of the serum IL-4 and IL-5 levels according to the serum IgE levels. Initial serum IL-4 and IL-5 levels were significantly higher in the high IgE group than in the normal IgE group. IgE, immunoglobulin E; IL, interleukin.

Fig. 2
Comparison of serum IgE levels according to the remission state. There were no changes in the median (range) levels of serum IgE according to the treatment state (nephrotic or remission phase) between the normal (p=0.667) and high IgE groups (p=0.131). IgE, immunoglobulin.

Fig. 3
Comparisons of the cytokines between nephrotic syndrome patients and healthy controls. (A) The serum levels of IL-4 in nephrotic syndrome patients with nephrotic phase were significantly higher than those of remission phase and controls. (B) The serum levels of IL-5 in nephrotic phase were also higher than those of normal controls. (C) The serum IL-10 levels did not show any significant differences in each group. (D) In the patients with nephrotic phase, the serum levels of TGF-β were significantly lower than those of remission phase or control group. This were calculated by using Kruskal-Wallis test Scheffe's post hoc comparison. The **means p<0.001, and *indicates p<0.05. IL, interleukin; TGF, transforming growth factor; NS, nephrotic syndrome.

Fig. 4
Correlations between serum cytokines and albumin levels. By linear regression analysis, (A) the serum IL-4 levels had a negative correlation with serum albumin (p=0.004), (B) the serum IL-5 levels had no correlation with serum albumin (p=0.113), (C) the serum IL-10 levels did not have a positive correlation with serum albumin levels (p=0.571), whereas (D) the serum TGF-β levels had (p<0.001). IL, interleukin; TGF, transforming growth factor.

References
1. Schachter AD. The pediatric nephrotic syndrome spectrum: clinical homogeneity and molecular heterogeneity. Pediatr Transplant. 2004. 8:344–348.

2. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981. 98:561–564.
3. Hardwicke J, Soothill JF, Squire JR, Holti G. Nephrotic syndrome with pollen hypersensitivity. Lancet. 1959. 1:500–502.

4. Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis. 2009. 54:945–953.

5. Salsano ME, Graziano L, Luongo I, Pilla P, Giordano M, Lama G. Atopy in childhood idiopathic nephrotic syndrome. Acta Paediatr. 2007. 96:561–566.

6. van den Berg JG, Weening JJ. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond). 2004. 107:125–136.

7. Cheung W, Wei CL, Seah CC, Jordan SC, Yap HK. Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol. 2004. 19:627–632.

8. Grimbert P, Audard V, Remy P, Lang P, Sahali D. Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant. 2003. 18:245–248.

9. Matsumoto K, Kanmatsuse K. Transforming growth factor-beta1 inhibits vascular permeability factor release by T cells in normal subjects and in patients with minimal-change nephrotic syndrome. Nephron. 2001. 87:111–117.

10. Souto MF, Teixeira AL, Russo RC, Penido MG, Silveira KD, Teixeira MM, et al. Immune mediators in idiopathic nephrotic syndrome: evidence for a relation between interleukin 8 and proteinuria. Pediatr Res. 2008. 64:637–642.

12. Le Berre L, Hervé C, Buzelin F, Usal C, Soulillou JP, Dantal J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005. 68:2079–2090.

13. Kanai T, Shiraishi H, Yamagata T, Ito T, Odaka J, Saito T, et al. Th2 cells predominate in idiopathic steroid-sensitive nephrotic syndrome. Clin Exp Nephrol. 2010. 14:578–583.

14. Araya C, Diaz L, Wasserfall C, Atkinson M, Mu W, Johnson R, et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2009. 24:1691–1698.

15. Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011. 139:314–320.

16. Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 1978. 13:159–165.
17. Dodig S, Richter D, Benko B, Zivcić J, Raos M, Nogalo B, et al. Cut-off values for total serum immunoglobulin E between non-atopic and atopic children in north-west Croatia. Clin Chem Lab Med. 2006. 44:639–647.

18. Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974. 2:556–560.

19. Lin CY, Lee BH, Lin CC, Chen WP. A study of the relationship between childhood nephrotic syndrome and allergic diseases. Chest. 1990. 97:1408–1411.

20. Tain YL, Chen TY, Yang KD. Implication of serum IgE in childhood nephrotic syndrome. Pediatr Nephrol. 2003. 18:1211–1215.

21. Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000. 105(2 Pt 2):S547–S558.

22. Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl. 2005. S62–S67.

23. Fuke Y, Endo M, Ohsawa I, Satomura A, Hidaka M, Fujita T, et al. Implication of elevated serum IgE levels in minimal change nephrotic syndrome. Nephron. 2002. 91:769–770.

24. Finkelman FD, Katona IM, Urban JF Jr, Holmes J, Ohara J, Tung AS, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988. 141:2335–2341.
25. Van Den Berg JG, Aten J, Chand MA, Claessen N, Dijkink L, Wijdenes J, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol. 2000. 11:413–422.

26. Daniel V, Trautmann Y, Konrad M, Nayir A, Schärer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997. 47:289–297.
27. Ohtomo Y, Fujinaga S, Hattori M. Suplatast tosilate dimethylsulfonium treatment for steroid-dependent nephrotic syndrome. Pediatr Int. 2005. 47:230–231.

28. Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009. 9:883–889.

29. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006. 212:8–27.

30. Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005. 16:1360–1370.

31. Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damião MJ, Cenedeze MA, et al. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009. 21:50–55.

32. Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, et al. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006. 17:2731–2741.

33. Shao XS, Yang XQ, Zhao XD, Li Q, Xie YY, Wang XG, et al. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr Nephrol. 2009. 24:1683–1690.

34. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998. 16:137–161.
35. Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med. 2006. 57:365–380.

36. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006. 69:213–217.

37. Reidy K, Kaskel FJ. Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol. 2007. 22:350–354.

38. Gekle M, Knaus P, Nielsen R, Mildenberger S, Freudinger R, Wohlfarth V, et al. Transforming growth factor-beta1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol. 2003. 552(Pt 2):471–481.

39. Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME. The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2007. 292:F1464–F1470.
40. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. 198:1875–1886.

41. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007. 317:256–260.

42. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007. 8:950–957.

43. Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 2001. 10:51–59.

44. Mottet C, Golshayan D. CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use. Swiss Med Wkly. 2007. 137:625–634.
45. Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, et al. Immune dysregulation in TGF-beta 1-deficient mice. J Immunol. 1994. 153:1936–1946.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download