Abstract
Purpose
The present visual and electromyographic study was designed to evaluate muscle fasciculations caused by succinylcholine in adults pretreated with either remifentanil 1.5 µg/kg or saline.
Materials and Methods
The effect of remifentanil on succinylcholine-induced muscle fasciculations was studied using a double-blind method in 40 adults. After i.v. pretreatment with either remifentanil 1.5 µg/kg (remifentanil group, n = 20) or an equivalent volume of i.v. saline (saline group, n = 20), patients were anaesthetized with a 2.0 mg/kg of i.v. propofol followed by i.v. succinylcholine 1.0 mg/kg. Intensity and duration of muscle fasciculation following i.v. succinylcholine administration were recorded. Electromyography (EMG) was used to quantify the extent of muscle fasciculation following i.v. succinylcholine injection. Myalgia was evaluated 24 hours after induction time. Serum potassium levels were measured five minutes after i.v. succinylcholine administration and creatine kinase (CK) levels 24 hours after induction time.
Results
Compared to saline treated controls, remifentanil decreased the intensity of muscle fasciculations caused by i.v. succinylcholine [fasciculation severity scores (grade 0 to 3) were 2/1/12/5 and 3/13/4/0 (patients numbers) in the saline group and the remifentanil group, respectively, p < 0.001]. The mean (SD) maximum amplitude of muscle action potential (MAP) by EMG was smaller in the remifentanil group [283.0 (74.4) µV] than in the saline group [1480.4 (161.3) µV] (p = 0.003). Postoperative serum CK levels were lower in the remifentanil group (p < 0.001). Postoperative myalgia was not different between the two groups.
Succinylcholine is still the accepted standard for rapid sequence intubation.1 Succinylcholine remains the drug of choice for rapid sequence intubation in the emergency department or intensive care unit, unless there is a contraindication to its usage.2,3 However, it has some disadvantages, such as fasciculation, postoperative myalgia, and an increase of serum creatinin kinase.4 Succinylcholine induced fasciculation may be a causative factor of an increase in intraocular, intracranial, or intragastric pressure.5 Also, even though there are a few recent studies that show otherwise,5,6 it is a widespread belief that fasciculation is the major cause of postoperative myalgia.7,8
Therefore, the prevention of succinylcholine induced muscle fasciculation may be an important strategy. Pretreatment with a small dose of a nondepolarizing muscle relaxant is perhaps the most popular technique for prevention of fasciculation. But with this technique, the risk of potentially serious adverse effects is not negligible.5
In children, fentanyl at 1 or 2 µg/kg inhibits muscle fasciculations as effective as non-depolarizing relaxants,9 and alfentanil 50 µg/kg, has been shown to inhibit muscle fasciculations caused by succinylcholine in children and young adults.10 Remifentanil can be a suitable alternative to alfentanil or fentanyl for attenuating muscle fasciculation by i.v. succinylcholine as it has a shorter duration of action. The present double blinded visual and electromyographic study was designed to evaluate muscle fasciculation, myalgia, and changes in serum potassium and creatine kinase (CK) concentrations caused by succinylcholine in adults pretreated with either remifentanil 1.5 µg/kg or saline.
After obtaining institutional Ethics Committee approval and informed consent, we studied 40 (ASA I or II) patients scheduled to undergo general anaesthesia for elective otolaryngological surgery of about one hour duration with tracheal intubation. This type of surgery was selected because it presents a minimal risk of surgically induced increases in serum CK. Patients with known cardiovascular, pulmonary, or metabolic diseases, and those with an impaired renal or hepatic function, morbid obesity, an age < 18 or > 65, or a history of drug abuse were excluded. In addition, patients with a neuromuscular disease and those with an anticipated airway difficulty or at increased risk of aspiration were also excluded. Using a random number table, patients were assigned to one of two groups by one anaesthesiologist, that is, to a saline group (normal saline 10 mL, n = 20) and remifentanil group (remifentanil 1.5 µg/kg diluted to 10 mL with normal saline, n = 20). Patients in both groups were premedicated with glycopyrrolate 0.004 mg/kg i.v., 10 min before anaesthesia.
Electromyography was used to quantify the extent of muscle fasciculation after i.v. succinylcholine administration. This was performed by measuring muscle action potentials (MAPs) at the biceps muscle in the arm without an i.v. route. MAPs were measured using disposable surface electrodes (11-mm diameter). For recording, the standard tendon-mid muscle belly montage was used to maximize MAP. Filters were set at 10 Hz and 10 kHz, and the sensitivity and sweep speed used were 100 µV per and 1 s per division. All subjects were examined using a Sapphire electromyography machine (Medelec, Surrey, UK), and recordings were performed by one investigator who was unaware of injection details.
In the operating theatre, a bolus of saline 10 mL or remifentanil 1.5 µg/kg diluted to 10 mL was administered over 30 s, 3 min after preoxygenation. When a bolus dose of i.v. saline or remifentanil was given, i.v. propofol 2 mg/kg was administered over 10 s. As soon as a patient lost consciousness (based on loss of response to command and loss of eyelash reflex), ventilation was attempted via a mask. Intravenous succinylcholine 1.0 mg/kg was given 60 s after completing the bolus administration of saline or remifentanil. Laryngoscopy and tracheal intubation were performed when visible muscle fasciculations stopped. The duration and severity of visible muscle fasciculation were assessed by one investigator, who was also unaware of the induction agent administered. Severity of fasciculation was graded as described by Mingus, et al.11: no fasciculations = 0; mild, fine fasciculations of the eyes, face, neck, or fingers without limb movement = 1; moderate fasciculations occurring on more than two sides or obvious limb movement = 2; and vigorous or severe, sustained and widespread fasciculations = 3.
Rocuronium 0.3 mg/kg was administered for muscle relaxation after tracheal intubation. Anaesthesia was maintained with 60% nitrous oxide in oxygen supplemented with 2-3 vol% sevoflurane. After tracheal intubation, patients were ventilated to maintain an end-tidal carbon dioxide level between 32 and 37 mmHg. Blood samples were drawn to determine serum potassium levels, without stasis, before the induction of anaesthesia, and five minutes after succinylcholine administration. Blood samples were taken to determine CK levels before induction and 24 hours after induction time.
Systolic blood pressures (SBPs) and heart rates (HR) were recorded before induction of anaesthesia, pre-intubation, and at 1, 2, 3, and 5 min after tracheal intubation. Hypotension (SBP < 80 mmHg for > 60 s) was treated with i.v. ephedrine in 5 mg increments; bradycardia (heart rate < 45 beat/min for > 60 s) was treated with i.v. atropine in 300 µg increments.12
All the patients were interviewed by an investigator unaware of the induction agent used 24 hours after anesthetic induction time postoperatively. Muscle pain not related to surgical intervention was graded as described by Harvey, et al.13: absence of muscle pain = no myalgia; minor stiffness limited to one area of the body = mild; muscle pain or stiffness noticed spontaneously by the patient, possibly requiring analgesic therapy = moderate; and generalized, severe, or incapacitating discomfort = severe. No intramuscular injection was administered during the perioperative period. Postoperative care was standardized for all patients. Pain related to surgical intervention was treated with ketorolac 30 mg i.v. as required.
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Sample size calculations were performed based on fasciculation scores, as described in a study that compared saline with alfentanil, with respect to its inhibitory effect on muscle fasciculations caused by succinylcholine in adults [median (range) fasciculation score from 0 to 3 were 2 (1-3) and 1 (0-2) in saline and alfentanil group, respectively].10 With a power of 80% and type 1 error of 5%, we calculated that 12 subjects were required per group. The Mann-Whitney U test was used to compare the severity of fasciculations in the two groups. The Student's t-test or the Paired sample t-test was used to analyze serum potassium and CK levels between and within groups. Student's t-test was used to analyze parametric data and Mann-Whitney U test for non-parametric data. Results are presented as mean (SD), and p values of < 0.05 were considered statistically significant.
All patients were included in the statistical analysis, and patient characteristics in the remifentanil and saline control groups were similar (p > 0.05) (Table 1). The intensity of visible muscle fasciculation in the remifentanil group was less than in the saline group (p > 0.01) (Table 2), whereas the incidences and durations of visible muscle fasciculation were similar in the two groups (p > 0.05).
Mild myalgia occurred in 2 patients (10%) in the saline group and in 1 patient (5%) in the remifentanil group.
Visible muscle fasciculations were not observed in two saline group patients but MAPs by electromyography (EMG) were detected in all 20 patients. In the remifentanil group, no visible muscle fasciculations were observed in three patients, and one of them had no MAP by surface EMG. Maximum MAP amplitudes were determined in all patients, and mean (SD) maximum MAP amplitude was smaller in the remifentanil group [283 (74.4) µV vs. 1480.4 (161.3) µV, p = 0.003]. The mean (SD) duration of MAPs in the remifentanil group was similar to that of the saline group [21.3 (3.9) s vs 26.8 (3.3) s, p > 0.05]. Typical EMG recordings are shown in Fig. 1.
Mean post-intubation serum potassium concentrations increased significantly from preoperative values in both groups (p < 0.001) (Table 3), but mean potassium concentrations at pre-induction and 5 min after tracheal intubation were not different in the two groups (p > 0.05). Post-operative mean CK levels increased significantly from their pre-operative values in both groups (p ≤ 0.001) (Table 3), and the mean post-operative CK level in the remifentanil group was lower than in the saline group (p < 0.001).
Two patients had hypotension (72-80 mmHg) at preintubation and at 2, 3, and 5 min after tracheal intubation in the remifentanil group. One of these two patients was treated with ephedrine 5 mg due to hypotension (SBP 72, 76 mmHg) for > 60 s. None of the patients had bradycardia (a heart rate < 45 beat/min for > 60 s) requiring treatment.
Two patients in each group were administered ketorolac (30 mg i.v.) within the first 24 hours postoperatively due to operation-related pain.
The present study shows that remifentanil 1.5 µg/kg decreases the intensity of visible muscle fasciculations and EMG activity caused by succinylcholine in adults. Maximum MAP amplitude provides an objective means of quantifying the severity of visible muscle fasciculations, but it was not utilized in previous similar studies.9,10
However, the incidence and duration of muscle fasciculation were not significantly different between the two groups, which is similar with the study that compared alfentanil with saline.10 The incidence and severity of myalgia also showed no significant difference. Only mild myalgia occurred in both group, 10% in the saline group, and 5% in the remifentanil group. Thus, the results of the present study correspond with studies reporting that there is no direct correlation between intensity of fasciculation and frequency of myalgia, and it is more likely that the etiology of myalgia is multifactorial.6,14-16 The results also correspond with the study reporting that the use of opioid during induction is not related with postoperative myalgia.5
Although the incidence of myalgia was low in both groups, the CK levels significantly increased in both groups when checked 24s hour after anesthetic induction time. But the CK level 24 hours after anesthetic induction time was significantly lower in the remfentanil group when compared to the saline group. It seems likely that this is related to the fact that the intensity of fasciculation was lower in the remifentanil group. These results also correspond to studies that reported that the incidence of myalgia does not correlate with CK, which is the biochemical marker of muscle damage.17,18 However, increases in serum potassium were not restricted by remifentanil. This is similar to that found in another study in which the muscle fasciculation was reduced but no difference in serum potassium was observed when pretreatment with d-tubocurarine was compared with no pretreatment.19
The mechanism of the inhibitory action of remifentanil on succinylcholine-induced muscle fasciculation remains unexplained. Morphine and nalorphine inhibit neuromuscular transmission in mammalian and amphibian neuromuscular preparations by impairing the release of acetylcholine.20-22 Also resting tension of the indirectly stimulated rat hemidiaphragm increases slowly in the presence of high concentrations of morphine.21 The animal studies indicate that morphine can significantly impair neurohumoral transmission at peripheral muscarinic receptors.23
Pretreatment with a small dose of non-depolarizing muscle relaxant, although widely used to reduce succinylcholine-induced fasciculation, posses many risks including heavy eyelids, blurred vision, diplopia, and difficulty in breathing or swallowing.11,24 It also causes the usage of a higher dose of succinylcholine in order to obtain optimal intubating condition which leads to a longer recovery and apnea period.25
Due to an extremely short elimination half-life, the plasma level of remifentanil decreases rapidly after discontinuation.26 According to our present study, the usage of remifentanil reduces the intensity of fasciculation caused by succinylcholine. This effect can be very useful when using succinylcholine in emergency situations by reducing the risk of regurgitation of gastric contents, since it is known that in adults the intensity of fasciculation is directly related with the increase of intragastric pressure in adults.27
Although remifentanil has the advantage of blocking the sympathetic activation during tracheal intubation,12,28 it can also cause adverse effects such as hypotension and bradycardia. Although hypotension occurred in 2 patients in the remifentanil group, bradycardia did not. It could be that pretreatment with glycopyrrolate prevented the adverse effect of remifentanil. Muscle rigidity, another well-known side effect of remifentanil, was also not observed. It is likely that prompt administration of propofol and succinylcholine blocked the incidence.
The dose of remifentanil selected (1.5 µg/kg) in the present study was based on the findings of previous studies in which remifentanil administered as a 1 µg/kg bolus followed by a 0.5 µg/kg infusion,12 or as a bolus of 1-2 µg/kg, effectively attenuated or blocked haemodynamic responses to laryngoscopy and intubation.28 However, the pretreatment of elderly or sick patients with remifentanil (1.5 µg/kg) should be carefully undertaken or be avoided because of possible hypotension and bradycardia.23 Further studies are required to optimize dosage regimens.
In conclusion, remifentanil 1.5 µg/kg attenuated intensity of muscle fasciculations by succinylcholine.
Figures and Tables
Fig. 1
Typical EMG recordings from the biceps of two patients pretreated with saline or remifentanil. EMG recordings during succinylcholine-induced muscle fasciculation in a patient pretreated with saline (A) or remifentanil (B). Arrows indicate times of i.v. succinylcholine (1.0 mg/kg) administration. Dotted arrow = maximum amplitude. EMG, electromyography.
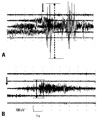
Table 2
Visible Muscle Fasciculations after I.V. Succinylcholine

Data are numbers of patients (%) and numbers of patients and mean (SD). Saline = the control group pretreated with saline. Remifentanil = the group pretreated with remifentanil 1.5 µg/kg. Fasciculation scores: 0 = none; 1 = mild; 2 = moderate; 3 = vigorous.
*p < 0.05 vs. fasciculation score of the saline group.
References
1. Morris J, Cook TM. Rapid sequence induction: a national survey of practice. Anaesthesia. 2001. 56:1090–1097.

2. Mallon WK, Keim SM, Shoenberger JM, Walls RM. Rocuronium vs. succinylcholine in the emergency department: a critical appraisal. J Emerg Med. 2009. 37:183–188.

3. Shoroghi M, Zahedi H, Farahbakhsh F, Sheikhvatan M, Abbasi A. The effect of thiopentone on severity and duration of succinylcholine-induced fasciculation. Clin Neuropharmacol. 2009. 32:94–96.

4. Kararmaz A, Kaya S, Turhanoglu S, Ozyilmaz MA. Effects of high-dose propofol on succinylcholine-induced fasciculations and myalgia. Acta Anaesthesiol Scand. 2003. 47:180–184.

5. Schreiber JU, Lysakowski C, Fuchs-Buder T, Tramèr MR. Prevention of succinylcholine-induced fasciculation and myalgia: a meta-analysis of randomized trials. Anesthesiology. 2005. 103:877–884.
6. Brodsky JB, Ehrenwerth J. Postoperative muscle pains and suxamethonium. Br J Anaesth. 1980. 52:215–218.

7. Wig J, Bali IM. Relation of precurarization to suxamethonium to provide ease of intubation and to prevent post-suxamethonium muscle pains. Can Anaesth Soc J. 1979. 26:94–98.

8. Tsui BC, Reid S, Gupta S, Kearney R, Mayson T, Finucane B. A rapid precurarization technique using rocuronium. Can J Anaesth. 1998. 45:397–401.
9. Lindgren L, Saarnivaara L. Effect of competitive myoneural blockade and fentanyl on muscle fasciculation caused by suxamethonium in children. Br J Anaesth. 1983. 55:747–751.

10. Yli-Hankala A, Randell T, Varpula T, Lindgren L. Alfentanil inhibits muscle fasciculations caused by suxamethonium in children and in young adults. Acta Anaesthesiol Scand. 1992. 36:588–591.

11. Mingus ML, Herlich A, Eisenkraft JB. Attenuation of suxamethonium myalgias. Effect of midazolam and vecuronium. Anaesthesia. 1990. 45:834–837.

12. Thompson JP, Hall AP, Russell J, Cagney B, Rowbotham DJ. Effect of remifentanil on the haemodynamic response to orotracheal intubation. Br J Anaesth. 1998. 80:467–469.
13. Harvey SC, Roland P, Bailey MK, Tomlin MK, Williams A. At randomized, double-blind comparison of rocuronium, d-tubocurarine, and "mini-dose" succinylcholine for preventing succinylcholine-induced muscle fasciculations. Anesth Analg. 1998. 87:719–722.

14. O'Sullivan EP, Williams NE, Calvey TN. Differential effects of neuromuscular blocking agents on suxamethonium-induced fasciculations and myalgia. Br J Anaesth. 1988. 60:367–371.
15. Leeson-Payne CG, Nicoll JM, Hobbs GJ. Use of ketorolac in the prevention of suxamethonium myalgia. Br J Anaesth. 1994. 73:788–790.

16. Ferres CJ, Mirakhur RK, Criag H, Browne ES, Clarke RS. Pretreatment with vecuronium as a prophylactic against post-suxamethonium muscle pain. Comparison with other non-depolarizing neuromuscular blocking drugs. Br J Anaesth. 1983. 55:735–741.

17. Maddineni VR, Mirakhur RK, Cooper AR. Myalgia and biochemical changes following suxamethonium after induction of anaesthesia with thiopentone or propofol. Anaesthesia. 1993. 48:626–628.

18. Laurence AS. Myalgia and biochemical changes following intermittent suxamethonium administration. Effects of alcuronium, lignocaine, midazolam and suxamethonium pretreatments on serum myoglobin, creatinine kinase and myalgia. Anaesthesia. 1987. 42:503–510.

19. McLoughlin C, Elliott P, McCarthy G, Mirakhur RK. Muscle pains and biochemical changes following suxamethonium administration after six pretreatment regimens. Anaesthesia. 1992. 47:202–206.

20. Frederickson RC, Pinsky C. Morphine impairs acetylcholine release but facilitates acetylcholine action at a skeletal neuromuscular junction. Nat New Biol. 1971. 231:93–94.

21. Pinsky C, Frederickson RC. Morphine and nalorphine impair neuromuscular transmission. Nat New Biol. 1971. 231:94–96.

22. Duke PC, Johns CH, Pinsky C, Goertzen P. The effect of morphine on human neuromuscular transmission. Can Anaesth Soc J. 1979. 26:201–205.

23. Cox BM, Weinstock M. The effect of analgesic drugs on the release of acetylcholine from electrically stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1966. 27:81–92.

24. Bruce DL, Downs JB, Kulkarni PS, Caplan LM. Precurarization inhibits maximal ventilatory effort. Anesthesiology. 1984. 61:618–621.

25. Eisenkraft JB, Mingus ML, Herlich A, Book WJ, Kopman AF. A defasciculating dose of d-tubocurarine causes resistance to succinylcholine. Can J Anaesth. 1990. 37:538–542.

26. Rosow C. Remifentanil: a unique opioid analgesic. Ansethesiology. 1993. 79:875–876.
27. Miller RD, Way WL. Inhibition of succinylcholine-induced increased intragastric pressure by nondepolarizing muscle relaxants and lidocaine. Anesthesiology. 1971. 34:185–188.

28. Barclay K, Kluger MT. Effect of bolus dose of remifentanil on haemodynamic response to tracheal intubation. Anaesth Intensive Care. 2000. 28:403–407.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download