Abstract
Purpose
Endoscopic thyroidectomy (ET) requires a proper working space for adequate visualization of anatomical structures and proper instrument manipulation. The purpose of this prospective study was to estimate the feasibility and safety of ET using an anterior chest wall approach without gas insufflation.
Materials and Methods
The working space was created under a direct and endoscopic view through a 3-cm incision on the anterior chest wall. A retracting device was then inserted to establish the working space, and subsequent procedures were performed endoscopically. All data were reviewed using a prospective database.
Results
We performed 30 ETs in patients with benign thyroid tumors from December 2003 to December 2005. The procedures were completed successfully in 29 patients (mean operative time: 160.6 min; range: 90-345 min). One patient with ET was converted to open thyroidectomy secondary to substernal extension of the tumor. None of the patients developed permanent postoperative hypocalcemia or recurrent laryngeal nerve paralysis. Three patients exhibited some degree of transient recurrent laryngeal nerve palsy.
New surgical techniques are developing rapidly, especially in the fields of laparoscopic or endoscopic surgery and robotic technology. These techniques have several well-known benefits, including minimal invasiveness, good cosmetic results and relatively low cost. Laparoscopic surgery was introduced early in the field of endocrine surgery, and laparoscopic-endoscopic adrenalectomy is now the first choice of treatment for adrenal gland tumors.1 The endoscopic surgical procedure for treating thyroid and parathyroid glands was introduced by Dr Gagner in 1995.2
Classical open thyroid surgery requires a long skin incision in the lower neck. This may lead to prominent scarring that can become hypertrophic or develop into a keloid. When a patient is contemplating thyroid surgery, he or she may delay their decision because of the scarring after surgery. Endoscopic thyroidectomy (ET) was introduced in an attempt to decrease scar length or prevent scars on the neck altogether, potentially making the patient more comfortable undergoing endoscopic surgery.3 However, not all endocrine surgeons are comfortable performing endoscopic surgery.
ET requires a proper working space for adequate visualization of anatomical structures and proper instrument manipulation. Many different techniques have been developed and applied to endoscopic and video-assisted thyroidectomy. These methods can be classified in terms of the ways by which they create a working space (gas insufflation or gasless) or introduce trocars (cervical, anterior chest wall, breast or axillary approaches). With regard to aesthetic issues, cervical, anterior chest wall, breast, and axillary approaches tend to cause less severe scarring.
The author began performing ET in December 2003. This surgeon had experience performing laparoscopic cholecystectomies for more than 10 years, in addition to laparoscopic appendectomies, laparoscopic adrenalectomies, and other laparoscopic procedures. The author performed ET with the anterior chest wall approach using a modified flap-lifting device introduced by Kim et al.4 We modified this technique, especially the spatula, for our own procedures (Fig. 1).
The purpose of this prospective study was to estimate the feasibility and safety of ET through the anterior chest wall without gas insufflation using a flap-lifting system.
Between December 2003 and December 2005, 30 patients underwent elective thyroid surgery using the ET technique with an anterior chest wall approach. Every patient had at least one definite ipsilateral mass proven to be a benign nodule according to fine needle aspiration cytology. 26 patients had nodular hyperplasia, one patient had a follicular neoplasm, two patients had cytological reports of atypia, and one patient had an unsatisfactory report. All of the tumors were evaluated in terms of their number, location and size by preoperative ultrasonography. Initial preoperative selection criteria for ET included a solitary thyroid nodule < 3cm in diameter, unilateral thyroid nodules < 3cm in diameter, small toxic thyroid nodules, thyroid cysts, absence of malignancy and absence of past surgical wounds in the operating area (Table 1).
All ET operations were performed and supervised by one surgeon, Dr. Cho. Our adapted operative technique combined the gasless chest approach of Kim et al.,4 the gas insufflation-axillary approach of Ikeda et al.,5 and the gasless axillary approach of Chung et al.6 The working space was created under direct and endoscopic view through the incision on the anterior chest wall (Fig. 2), which created subplatysmal flaps. Working space for the thyroidectomy was maintained with a flap-lifting system, a modification of Kim's device (Fig. Fig. 1 and Fig. 3).
After the working space was created, all procedures were performed in an endoscopic manner. One or two additional incisions were created to introduce one or more 5-mm trocars. Ultrasound was used to divide named and innominate vessels of the thyroid gland. All dividing procedures for the thyroidectomy were performed with an electric surgical device and ultrasonically activated shears. The safety and effectiveness of these shears during thyroidectomy was confirmed in a previous randomized study conducted by Voutilanien et al.7 After creating the working space, the thyroid gland was exposed between the strap muscles of the sternohyoid and sternothyroid muscles. Surgical clips were used as needed to control vascular bleeding. All procedures performed on the thyroid gland were ipsilateral thyroid lobectomies. All data were reviewed in a prospective database.
Patients were prepared for ET under general anesthesia. After induction the patient was placed in a supine position, the neck was extended slightly with a pillow beneath the shoulder. The head was then rotated towards the lesion to flex the strap muscles (e.g., slight rotation to the right for a lesion on the right side). A 3-cm oblique skin incision was made in a midclavicular line on the anterior chest wall, taking into consideration the wearing pattern of the patient. These areas were then dissected sharply and gently underneath the platysma muscle, advancing upwardly and medially from the incision to the thyroid area and across the medial border of the sternocleidomastoid muscle. Careful handling was required for dissection over the sternocleidomastoid muscle since resistance was expected in this area. A 5-mm trocar was inserted lateral to the skin incision. The margins of the incised opening were then covered with a silastic material (usually a silastic drain) to minimize burn injury, which can cause a keloid scar. The lifting device was subsequently installed, and a 30˚ 5-mm endoscope was inserted through the 5-mm port. Additional procedures to create adequate working space for thyroid gland dissection were achieved with endoscopic scissors or hook by electrical cauterization. The instruments for this technique were introduced through the main incision. The lifting device had a spatula-type retractor, with a hole at the midline allowing connection of a suction tube. We employed continuous suctioning of vaporized steam and smoke during the dissection. Finally, the working space was created from the anterior chest to the thyroid cartilage level above the strap muscle by lifting the subplatysmal skin flap. The working space was extended laterally to the lateral border of the sternocleidomastoid muscle and medially just crossing the midline colli.
After creating the working space, a dissection was made between the strap muscles. The thyroid gland was then exposed and dissected from the inferior pole, revealing the inferior thyroid vessels. The branches of the inferior thyroid vein were divided with an ultrasonically activated scalpel (Harmonic Scalpel; Ethicon Endo-Surgery, New Brunswick, NJ, USA) and the thyroid gland was retracted medially with endoscopic forceps. It was sometimes easy to divide the isthmus before proceeding to the posterior and lateral aspects of the gland with the ultrasonically activated scalpel. The inferior thyroid artery and the middle thyroid vein were skeletonized and divided with an ultrasonically activated scalpel after identification of the recurrent laryngeal nerve. The superior thyroid artery and vein were identified and divided individually, and the gland was retracted medially and inferiorly. The thyroid was then separated from the trachea, taking great care to divide the Ligament of Berry. A thyroid tumor involving the parenchyma was divided at the isthmus, and the resected specimen was retrieved through the incisional opening at the anterior chest wall.
After completion of hemostasis, the strap muscles were approximated with absorbable sutures. A 5-mm closed-suction drain was inserted through the new incision wound at the lower portion of the operative space. The incision wounds were closed with layer-by-layer sutures.
From December 2003 to December 2005, we performed ETs in 30 patients with thyroid tumors diagnosed with a benign tumor before surgery. Twenty-nine patients were female and one patient was male. The mean age of the patients was 38.4 ± 10.4 years (range: 20-58 years) (Table 2).
All patients were selected based on ultrasonographic and cytological evaluations prior to surgery. The characteristics and sizes of the tumors were evaluated by ultrasonography. The largest diameter of each tumor was measured, and patients were selected according to a criterion of < 30mm for the largest diameter (Table 2). However three patients with larger tumors were also included (diameters of 31mm, 35mm, and 36mm) because these patients demanded endoscopic surgery to avoid leaving a scar on the neck.
Our study included one patient with three tumors, 10 patients with two tumors, and 19 patients with a single tumor. 16 patients had tumors located in the right lobe of the thyroid and 14 patients had tumors in the left lobe. All tumors were ipsilateral.
Preoperative cytology reports showed 16 cases of nodular hyperplasia, two of atypia, one of follicular neoplasm, and one unsatisfactory report. The two patients with atypia were included in the selection based on other diagnostic reports. The final pathology of both patients was ultimately Hashimoto's thyroiditis (Table 2).
Twenty-nine of 30 patients received complete and successful hemithyroidectomies, with a mean operative time of 160.6 ± 49.6min (range: 90-345 min) (Table 2). One case of ET was converted to open conventional thyroidectomy due to substernal tumor extension that was not detected before surgery, despite ultrasonographic examination. Anesthesia was maintained during the surgery for 202.6 ± 51.0min (range: 120-385 min). Time required for preparation and evacuation was approximately 40 minutes, suggesting that installation of the endoscopic tools was not a complicated procedure.
The mean estimated blood loss was 54.8 ± 76.6cc (range: 5-400cc). The mean specimen weight including the tumor and ipsilateral thyroid was 14.3 ± 5.6g (range: 7-30g) (Table 2).
Collection via Jackson-Pratt drain was monitored before the drain was removed, with a total drainage of 79.3 ± 33.9cc (range: 50-180cc). No specific complications related to bleeding occurred postoperatively. Patients were discharged 4.6 days post-op on average (range: 3-6 days) (Table 2).
Operative complications manifested in three patients as transient recurrent laryngeal nerve palsy. One patient had mild hoarseness and aspiration, and two patients experienced transient hoarseness. Wound issues such as hypertrophic scars or keloids (four patients) and paresthesias (one patient) were also observed in the main incision area. One patient developed a delayed incisional wound infection in the form of a stitch abscess. A second-degree burn was caused by electrical insult during creation of the skin flap; however, the wound healed well without any secondary complications (Table 2).
The final pathology of the tumors included nodular hyperplasia (n = 28), Hashimoto's thyroiditis (n = 3), chronic lymphocytic thyroiditis (n = 4), papillary carcinoma (n = 4), and follicular carcinoma (n = 1) (Table 2). The pathology revealed malignancy in five patients; of whom two had papillary carcinoma, two had papillary thyroid microcarcinoma, and one had minimally invasive follicular carcinoma. One patient with papillary carcinoma had an additional operation to complete the thyroidectomy using a conventional open method. The other patients diagnosed with malignant pathologies are being closely monitored.
In the past decade new technologies have developed in all surgical specialties, including endocrine surgery. Laparoscopic adrenalectomy is now the preferred surgery for benign adrenal tumors. Since its introduction for the parathyroid gland, endoscopic surgery has become the predominant method of surgery for the thyroid and parathyroid glands. Some experts now perform ET for well-differentiated thyroid carcinoma and Graves' disease,8,9 in addition to benign thyroid tumors.
Despite its cosmetic advantages, ET has some limitations for surgeons with little experience in this practice. It is difficult to create an adequate working space in such a narrow area. Additionally, it is challenging to orient oneself to anatomical structures using monitor images from the videoscope (endoscope). Surgical trainees must learn these techniques under the supervision of an experienced surgeon.
The techniques used in endoscopic surgery are classified in terms of creating working space (gas insufflation or gasless) and introducing the trocars (cervical, anterior chest wall, breast, or axillary approaches). With regard to cosmesis, wounds are least noticeable after surgery via axillary approach, followed by breast, anterior chest wall, and cervical approaches.10 However, because each person has his or her own style of clothing, the desired amount of skin exposure may differ between patients. Surgeons may adopt any of these techniques based on the individual patient, as all are convenient and safe.
This author chose an anterior chest wall approach, because ET is not performed frequently and videoscopic anatomy is unfamiliar to endocrine surgeons. The first ET was performed through an axillary incision by Yoon et al.6; however the relatively long route to the operating working space from the skin incision (axilla) made this approach inconvenient for the surgeon and other personnel. Therefore, the author modified the method by making an incision in the anterior chest wall. This allowed the surgeon to directly visualize the surgical anatomy, feel structures by finger-touch through the incision, and allow trainee surgeons to adapt to the procedure more easily.
During endoscopic procedures with gas insufflation, the videoscopic view can be obscured secondary to narrowing of the working space when suctioning steam, smoke, and blood from the operative field. Therefore, in this study, the working space was maintained by a retracting system using a gasless technique. After choosing the method of surgery, the author modified a part of the flap-lifting system. The spatula for the retraction of the skin flap was narrowed to 2.5cm wide. A hole was added for the connection of a suction tube, allowing continuous suction and a clear visual field throughout the operation. This technique of ET may also produce good cosmetic results. Although it involves a complicated technique, ET results in a minimally perceptible wound on the chest wall. However, some patients developed hypertrophic scars or even keloids. Thus some surgeons prefer to remove a large mass via an incision no greater than 8cm in diameter. However, this procedure is very difficult to perform for masses greater than 3cm in diameter. Other complications with these wounds postoperatively include numbness, wound discomfort, and mild pain with neck movement or swallowing. In this study the only postoperative complication was three cases of transient recurrent laryngeal nerve palsy. The ability to identify and preserve the recurrent laryngeal nerve during ET has subsequently helped prevent transient hoarseness. During this procedure, extreme care must be taken when dissecting the recurrent laryngeal nerve from direct and thermal injury by electrocautery and ultrasonic scalpel. Wound pain and discomfort were relieved one or two months after the operation, and no late complications were observed. The cosmetic advantages are self- evident; every patient was satisfied with the procedure in terms of cosmesis and completeness of the thyroidectomy procedures (Fig. 4A and 4B).
The operation time of ET is typically longer than a conventional open thyroidectomy. The axillary approach has a longer operation time than even the other ET procedures.3,11 ET through the anterior chest wall was performed in a mean operation time of 160 min, and resulted in a mean estimated blood loss of 54.8cc. One case with an extremely long operation time of 345 min was due to poorly functioning videoscopic equipment, which was exchanged with another system. The author adopted this technique after personal communications with Dr Kim (anterior chest wall- gasless),4 Dr Chung (axillary-gasless),6 and Dr Park (breast-gas insufflation).12 Gasless ET via an anterior approach for benign thyroid tumors may be convenient for surgeons who practice ETs less frequently, or have little to no experience with endoscopic procedures. This method of ET provides a consistent working space without collapse during suction, unlike the gas-insufflation method. The surgeon also has the opportunity to examine the anatomy by palpation or direct visualization through the main incision.
In conclusion, the gasless ET via the anterior chest wall approach is a safe and feasible procedure for the treatment of benign thyroid tumors. It provides appropriate cosmetic results, even with small volume thyroidectomies. This technique might be recommended for novice surgeons when performing ET. However, precise determination of the location and characteristics of the tumor prior to surgery is essential.
Figures and Tables
Fig. 1
A flap-lifting system. The spatula should be introduced into the main incision on the anterior chest wall, lifting the skin flap.

Fig. 2
Schematic drawing of the working space for gasless endoscopic thyroidectomy via the anterior chest wall approach.
Fig. 3
Operative field after installation of all devices. One surgeon handles the endoscope and the other uses the endoscopic forceps as dissectors, clamps, or for other functions.
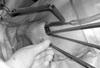
Fig. 4
(A) Scars on the ipsilateral anterior chest wall one month post-op. (B) Scars are not visible when the patient is clothed.

Table 2
Demographic characteristics and results of 30 consecutive gasless endoscopic thyroidectomies through an anterior chest wall approach

FNAC, fine needle aspiration cytology; F, female; M, male; L, left; R, right; NA, not applicable; RLN, recurrent laryngeal nerve; NH, nodular hyperplasia; MiFC, minimally invasive follicular carcinoma; CLT, chronic lymphocytic thyroiditis; PTMC, papillary thyroid microcarcinoma; PTC, papillary thyroid carcinoma.
References
2. Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996. 83:875.

3. Ikeda Y, Takami H, Sasaki Y, Takayama J, Niimi M, Kan S. Comparative study of thyroidectomies: Endoscopic surgery versus conventional open surgery. Surg Endosc. 2002. 16:1741–1745.
4. Kim JS, Kim KH, Ahn CH, Jeon HM, Kom EG, Jeon CS. A clinical analysis of gasless endoscopic thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2001. 11:268–272.

5. Ikeda Y, Takami H, Sasaki Y, Takayama J, Niimi M, Kan S. Clinical benefits in endoscopic thyroidectomy by the axillary approach. J Am Coll Surg. 2003. 196:189–195.

6. Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech. 2006. 16:226–231.

7. Voutilainen PE, Haglund CH. Ultrasonically activated shears in thyroidectomies: a randomized trial. Ann Surg. 2000. 231:322–328.
8. Kitagawa W, Shimizu K, Akasu H, Tanaka S. Endoscopic neck surgery with lymph node dissection for papillary carcinoma of the thyroid using a totally gasless anterior neck skin lifting method. J Am Coll Surg. 2003. 196:990–994.

9. Yamamoto M, Sasaki A, Asahi H, Shimada Y, Sato N, Nakajima J, et al. Endoscopic subtotal thyroidectomy for patients with Graves' disease. Surg Today. 2001. 31:1–4.

10. Yeung GH. Endoscopic thyroid surgery today: a diversity of surgical strategies. Thyroid. 2002. 12:703–706.

11. Yamamoto M, Sasaki A, Asahi H, Shimada Y, Saito K. Endoscopic versus conventional open thyroid lobectomy for benign thyroid nodules: a prospective study. Surg Laparosc Endosc Percutan Tech. 2002. 12:426–429.

12. Park YL, Han WK, Bae WG. 100 cases of endoscopic thyroidectomy: breast approach. Surg Laparosc Endosc Percutan Tech. 2003. 13:20–25.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download