Abstract
Infantile spasm is an age-related refractory epilepsy. Topiramate is a new anticonvulsant with multiple mechanisms of action, and it may be effective for treating pediatric epilepsies. To evaluate the efficacy and tolerability of first-line topiramate treatment for infantile spasm, 20 patients received topiramate monotherapy during this study. They were treated with an initial dose of 1 mg/kg/day, with a progressive titration of 1 mg/kg a week until their spasms were controlled and a maximum dose of 12 mg/kg/day was achieved. The evaluation of the treatment efficacy was based on the spasm frequency data that was obtained by the scalp and video-EEG, and by the parental count of spasm. Thirty percent of the subjects became spasm-free during the study. Six of 20 subjects (30%) had cessation of spasm and disappearance of hypsarrhythmia as seen via the video EEG; four (50%) of eight idiopathic patients had a response, whereas two (17%) of 12 patients with symptomatic infantile spasm responded. Seventy of the patients, including the spasm-free patients, had a reduction in their seizure frequency of more than 50%, and 10% of the patients had a reduction in their seizure frequency of less than 50%. The clusters of spasm frequency decreased from 10.6 ± 8.5 to 3.5 ± 1.4 clusters/day. Topiramate is effective and tolerated in those patients suffering from infantile spasm. Our results suggest that this drug should be considered as a new first-line drug for treating infantile spasm.
Infantile spasm is a catastrophic childhood epilepsy syndrome1 that was first described by West in 1841.2 The combination of infantile spasm, a characteristic electroencephalographic pattern and psychomotor retardation in the majority of patients is known as West Syndrome.3
For the management of infantile spasm, ACTH/steroid or vigabatrin (VGB) are widely and frequently as the first-line of treatment.4,5 Because the usefulness of these drugs is limited by the occurrence of side effects, scientists are searching for new antiepileptic drugs to treat infantile spasm.6-12
Topiramate (TPM) is a drug with multiple mechanisms of action, including the state-dependent inhibition of sodium channels, the potentiation of γ-aminobutyric acid (GABA)-induced chloride influx, the blockade of glutamate related excitatory neurotransmission, and the inhibition of carbonic anhydrase.13 TMP has been used as a monotherapy and adjunctive treatment of partial-onset and generalized tonic-clonic seizure in adults,14,15 and for partial seizures in children.16 Additionally, TPM is effective when used as adjunctive therapy for the management of Lennox-Gastaut Syndrome.17
Previous studies have suggested that TPM is effective for the treatment of infantile spasm when it is used as an add-on therapy.18 Although a pilot study attempted to evaluate TPM as a monotherapy for infantile spasm,19 there remains a need for a more effective first-line treatment with reduced adverse effects. The aim of this study was to assess the effectiveness and tolerability of TPM when used as the initial treatment for patients with newly diagnosed infantile spasm.
Twenty patients with newly diagnosed infantile spasm were treated with TPM between January 2001 and December 2003. This study included 17 male and 3 female subjects between 3 and 24 months of age. Subjects were exhibited an average of more than one cluster of spasms per day over one month before the start of TPM therapy to establish seizure frequency.
The following criteria were used to establish the diagnosis of infantile spasm: (1) seizures were characterized by axial muscle flexion, extension, or mixed spasms. (2) electroencephalographic findings demonstrated hypsarrhythmia, modified hypsarrhythmia, or multifocal spike-wave discharges.
To ensure that we obtained the informed consent of the patients' families, we provided them with detailed information about the standard treatment options available for infantile spasm. We also provided the families with information on the efficacy and potential side effects of TPM. Additionally, we educated them about their children's spasms and asked them to maintain daily diaries to document the occurrences of the spasms. Efficacy evaluation was based on cluster frequency data obtained from using 24-hour video electroencephalogram (EEG), scalp EEG, and from analyzing the daily diary of the children's seizure occurrence. EEG recordings were performed upon entry into this study and absence of clusters of spasm at that time, or at the end of the trial. TPM was given at an initial dose of 1 mg/kg per day, with a progressive titration of 1 mg/kg a week until spasms were controlled, the maximal tolerated dose was reached, or the maximal dose of 12 mg/kg per day was achieved. At 12 weeks after the initiation of TPM monotherapy, we analyzed all patients to measure the effectiveness and tolerability of TPM. Evaluation of effects was based on frequency of the cluster of spasms, with response defined as cessation of spasms and disappearance of the hypsarrhythmic EEG pattern. Adverse effects were monitored and recorded. We obtained blood and urine samples for routine laboratory examination following the study period. The routine laboratory examination included complete blood cell and platelet counts, electrolyte analysis, urinalysis, and assays for liver and renal function. Statistical evaluation was performed using the Mann-Whitney test. A p values < 0.05 was considered statistically significant.
The median age for infantile spasm onset was 6.5 months (range, 3 to 24 months), and the median follow-up duration of spasms after starting TPM monotherapy was 15.7 months (range, 4 to 30 months). Flexor spasms were the most comment type of spasm (Table 1).
Regarding the etiologic factor, eight (40%) patients had cryptogenic epilepsy. Among the twelve (60%) symptomatic cases, hypoxic-ischemic encephalopathy (HIE) was the most common underlying etiologic factor, followed by central nervous system anomaly (Table 2).
Overall, six (30%) patients became spasm-free for an average of 51 ± 24 days (range, 22 to 87 days) at TPM doses ranging from 4 mg/kg to 12 mg/kg per day. The mean dose of TPM during stabilization was 9.1 ± 3.1 mg/kg per day. Spasm frequency decreased 67% from the baseline to the stabilization phase (Table 3). Seventy percent of the patients who received TPM, including the six spasm-free, achieved a ≥ 50% reduction in spasm frequency compared to baseline (Table 3). Maintenance doses ranged between 9 and 12 mg/kg per day. One patient titrating 5 mg/kg per day had an increase in spasm frequency. Overall, 6 of 20 patients became free of spasms and hypsarrhythmia. Four (20%) of eight cryptogenic infantile spasm patients became spasm free, whereas 2 (10%) of 12 symptomatic patients responded. TPM appeared to be more effective with cryptogenic than symptomatic epilepsy (Table 4). Of the six responders, however, there was one (17%) had a relapse of spasms within four weeks. The four patients with normalized EEGs remained spasm-free, while one infant continued to have an abnormal EEG pattern with persisting focal spike discharges, though without relapse of spasms.
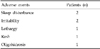
Five (25%) patients displayed adverse effects during titration or stabilization or both. Adverse effects included sleep disturbance, irritability, lethargy, rash, and oligohidrosis (Table 5). We observed one case of oligohidrosis in a 6-month-old female treated with TPM. At that time, one month after TPM introduction, the TPM dose was titrated to 4 mg/kg per day. TPM was gradually tapered, and subsequently her symptoms completely subsided. Laboratory tests revealed this patient had normal hematology and normal liver and renal function.
Infantile spasm is a catastrophic childhood epilepsy syndrome1 that is characterized by flexor or extensor spasm, hypsarrhythmia and psychomotor regression. Despite the use of a variety of therapies for infantile spasm, complete seizure control is difficult to achieve and the optimal treatment remains uncertain at best. The efficacy of the various drugs used in the treatment of infantile spasm is often difficult to determine because of the short follow-up periods of the studies and the lack of data on the long-term effects.
Adrenocorticotrophic hormone (ACTH) was first reported in the 1950s to have rapid effects on spasms.20 For approximately 50 years, ACTH has been widely used as the drug of choice for the treatment of infantile spasm.4 ACTH induces a reduction or cessation of spasms and the disappearance of hypsarrhythmia on the EEG in approximately 50-75% of patients.21-23 Despite its efficacy, ACTH has been associated with side effects, including hypertrophic cardiomyopathy, hypertension, infection, and electrolyte disturbance.6-10 Furthermore, the relapse rate ranges from 32% to 87% during corticotropin therapy.24
Although valproate and the benzodiazepines have therapeutic effects, conventional antiepileptic drugs are usually ineffective in reducing seizure activity.25-27 Furthermore, adverse effects on the fetus, including irreversible hepatotoxicity, have been associated with the use of valproate.28 Benzodiazepines usually require additional medications, the use of which can cause defective swallowing and a tendency for aspiration with higher doses.29 Thus, there is a need for a more effective first choice drug with reduced side-effect. Because of recent advances in the understanding of the basic neuropathology, neuropharmacology, and neurophysiology of epilepsy, several new antiepileptic drugs have been developed and use in therapeutic trials.
Since vigabatrin (VGB) was first reported as an add-on therapy for resistant infantile spasm in 1991,30 it has been considered as another drug to use as first-line therapy for infantile spasm in many countries outside the United States.5,31 In an open-label study of VGB as a first-line treatment for infantile spasm, 26% of the subjects became spasm free.32 Another study showed that 64% of the subjects had a clinical response that included the complete cessation of spasms.33 In our study, as in the above earlier studies, TPM has shown a similar reduction of spasm response rates (30% of the subjects became spasm-free, and 40% of the subjects experienced more than a 50% reduction of spasm) as compared with VGB. Several reports have demonstrated that VGB has excellent efficacy, with complete control occurring in about 95% to 100% of the patients with infantile spasm due to tuberous sclerosis.34,35 However, recent reports of visual field defects associated with VGB treatment may limit its utility.11,12,36
Some open-label trials on instituting monotherapy or adjunctive therapy with the new antiepileptic drugs, including lamotrigine (LTG),37 felbamate,38 levetiracetam,39 zonisamide (ZNS),40 and topiramate,19 have reported preliminary evidence for the efficacy of these drugs in treating infantile spasm. However, there are only limited reports on using LTG, felbamate and levetiracetam in patients with infantile spasm. LTG was reported to be effective as an add-on therapy for 30 patients with infantile spasm who were resistant to several other drugs.41 However, this condition may progress to the potentially life-threatening Stevens-Johnson syndrome or toxic epidermal necrolysis.42 Because of this possibility, one recent report suggested the effectiveness of using low-dose LTG in 3 patients.43 Felbamate may be effective for infantile spasms, as a response was noted in three of four patients. Yet after reports of aplastic anemia44 and hepatotoxicity,45 the use of felbamate as a treatment option for infantile spasm may be limited. One case report described the successful use of levetiracetam therapy in an 11-month-old infant with infantile spasms. The dose was gradually increased to 15 mg/kg/day up to a dosage of 60 mg/kg per day.39 ZNS has been used in Japan since 1989, and several reports have suggested that ZNS can be effective as the initial treatment for infantile spasm.40,46,47 However, there is only limited published data concerning the use of ZNS for infants with infantile spasm.
The data on using TPM as a potential first-line therapy for infantile spasm are relatively scarce. Glauser et al. first reported that TPM might be effective as a first-line therapy for infantile spasm.19 The dose titration schedule of that study was 2-4 times faster, it was rapidly titrated over four weeks, and it was increased up to a dosage of 24 mg/kg per day, a greater dose increase than in previous pediatric TPM studies.16,48,49 In those studies, the mean dose of TPM during stabilization was 15.0 ± 5.7 mg/kg per day. These studies showed that 45% of patients became spasm free and 9 of 11 patients experienced a ≥ 50% reduction in spasm frequency. The slow dose titration of TPM allowed assessment of the patients' responses and increased patient tolerability to the drug.50 The dose range of TPM is 1.2-12 mg/kg once or twice daily, and a slow titration of 0.5-2 mg/kg/day every 2 weeks was well tolerated and resulted in only mild to moderate adverse effects.51 In our study, TPM was initiated at 1 mg/kg per day and it was slowly titrated over 12 weeks up to a dosage of 12 mg/kg per day. Our results showed that the mean dose of TPM during stabilization was 9.1 ± 3.1 mg/kg per day, and 70% of the subjects achieved a ≥ 50% reduction in spasm frequency, including six patients who became spasm-free. These results suggested that the maximal therapeutic dose and mean dose of stabilization are lower than those reported by previous studies. Despite these differences, there were no differences in the observed effects of TPM between the reports.
Although several reports displayed findings contrary to the current textbook findings, patients with cryptogenic infantile spasm responded better to TPM than did the symptomatic patients.46,52 Our data is similar to above the textbook features. Four of eight cryptogenic patients (50%) had a normalized EEG and were spasm-free, whereas only 2 of 12 symptomatic patients (17%) responded. The observed efficacy of TPM in our study is similar that observed for VGB in cryptogenic and symptomatic patients (62% and 29%, respectively).5
The reported side effects of TPM include central nervous system problems,53 behavioral and cognitive problems,53 gastrointestinal effects,54 weight loss,55 acute angle-closure glaucoma,56 oligohydrosis57 and kidney stones.58 Although five patients in this study manifested adverse effects, they were mild to moderate in severity. The side effect profile mainly involved the central nervous system, and these problems were generally short-lived. None of the patients in our study developed weight loss, acute angle-closure glaucoma, or kidney stones.
In conclusion, our results demonstrate that TPM might be both effective and well tolerated as a first-line therapy for infantile spasm. We believe that slow dose titration and a low maximal dose of TPM should be considered when starting monotherapy in patients with infantile spasm. Yet further studies involving a larger numbers of patients are warranted and will be required to determine the long-term outcome of the TPM responders.
References
1. Shields WD. West's syndrome. J Child Neurol. 2002. 17:Suppl 1. S76–S79.
2. West WJ. On a peculiar form of infantile convulsions. Lancet. 1841. i:724–725.
3. Commission on Classification and Terminology of the International League against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989. 30:389–399.
4. Hancock E, Osborne JP, Milner P. The treatment of West syndrome: a Cochrane review of the literature to December 2000. Brain Dev. 2001. 23:624–634.
5. Fejerman N, Cersosimo R, Caraballo R, Grippo J, Corral S, Martino RH, et al. Vigabatrin as a first-choice drug in the treatment of West syndrome. J Child Neurol. 2000. 15:161–165.
6. Young RS, Fripp RR, Stern DR, Darowish C. Cardiac hypertrophy associated with ACTH therapy for childhood seizure disorder. J Child Neurol. 1987. 2:311–312.
7. Bobele GB, Ward KE, Bodensteiner JB. Hypertrophic cardiomyopathy during corticotropin therapy for infantile spasm. A clinical and echocardiographic study. Am J Dis Child. 1993. 147:223–225.
8. Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983. 24:135–158.
9. Shamir R, Garty BZ. Pneumocystis carinii pneumonia associated with adrenocorticotrophic hormone treatment for infantile spasms. Eur J Pediatr. 1992. 151:867.
10. Riikonen R, Simell O, Jaaskelainen J, Rapola J, Perheentupa J. Disturbed calcium and phosphate homeostasis during treatment with ACTH of infantile spasms. Arch Dis Child. 1986. 61:671–676.
11. Hilton EJ, Cubbide RP, Hosking SL, Betts T, Comaish IF. Patients treated with vigabatrin exhibit central visual function loss. Epilepsia. 2002. 43:1351–1359.
12. Jensen H, Sjo O, Uldall P, Gram L. Vigabatrin and retinal changes. Doc Ophthalmol. 2002. 104:171–180.
13. Rogawski MA, Porter RJ. Antiepileptic drugs: pharmacological mechanisms and clinical efficacy with consideration of promising development stage compounds. Pharmacol Rev. 1990. 42:223–286.
14. Ben-Menachem E, Henriksen O, Dam M, Mikkelsen M, Schmidt D, Reid S, et al. Double-blind, placebo-controlled trial of topiramate as add-on therapy in patients with refractory partial seizure. Epilepsia. 1996. 37:539–543.
15. Biton V, Montouris GD, Ritter F, Riviello JJ, Reife R, Lim P, et al. Topiramate YTC Study Group. A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Neurology. 1999. 52:1330–1337.
16. Elterman RD, Glauser TA, Wyllie E, Reife R, Wu SC, Pledger G. Topiramate YTC Study Group. A double-blind, randomized trial of topiramate as adjunctive therapy for partial-onset seizures in children. Neurology. 1999. 52:1338–1344.
17. Glauser TA, Levisohn PM, Ritter F, Sachdeo RC. Topiramate YL Study Group. Topiramate in Lennox-Gastaut syndrome: open-label treatment of patients completing a randomized controlled trial. Epilepsia. 2000. 41:Suppl 1. S86–S90.
18. Watemberg N, Goldberg-Stern H, Ben-Zeev B, Berger I, Straussberg R, Kivity S, et al. Clinical experience with open-label topiramate use in infants younger than 2 years of age. J Child Neurol. 2003. 18:258–262.
19. Glauser TA, Clark PO, Starwburg R. A pilot study of topiramate in the treatment of infantile spasms. Epilepsia. 1998. 39:1324–1328.
20. Sorel L, Dusaucy-Bauloye A. A propos de 21 cas d'hypsarrhythmia de Gibbs. Son traitment spectaculaire par 1'ACTH. Acta Neurol Psychatr Belg. 1958. 58:130–131.
21. Ito M, Aiba H, Hashimoto K, Kuroki S, Tomiwa K, Okuno T, et al. Low-dose ACTH therapy for West syndrome: initial effects and long-term outcome. Neurology. 2002. 58:110–114.
22. Harchovy RA, Frost JD, Kellaway PR, Zion TE. A controlled study of ACTH therapy in infantile spasms. Epilepsia. 1980. 21:631–636.
23. Harchovy RA, Frost JD Jr, Kellaway P, Zion TE. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983. 103:641–645.
24. Singer WD, Rube EF, Haller JS. The effect of ACTH therapy upon infantile spasms. J Pediatr. 1980. 96:485–489.
25. Bachman DS. Use of valproic acid in treatment of infantile spasm. Arch Neurol. 1982. 39:49–52.
26. Watanabe K. Medical treatment of West syndrome in Japan. J Child Neurol. 1995. 10:143–147.
27. Haines ST, Casto DT. Treatment of infantile spasms. Ann Pharmacother. 1994. 28:779–791.
28. Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB. Valproic acid hepatitis fatalities: a retrospective review. Neurology. 1987. 37:379–385.
29. Mikati MA, Lepejian GA, Holmes GL. Medical treatment of patients with infantile spasms. Clin Neuropharmacol. 2002. 25:61–70.
30. Chiron C, Dulac O, Beaumont D, Palacios L, Pajot N, Mumford J. Therapeutic trial of vigabatrin in refractory infantile spasms. J Child Neurol. 1991. 6:Suppl 2. S52–S59.
31. Aicardi J, Mumford JP, Dumas C, Wood S. Sabril IS Investigator and Peer Revies Groups. Vigabatrin as initial therapy for infantile spasms: a European retrospective survey. Epilepsia. 1996. 37:638–642.
32. Granstrom ML, Gaily E, Liukkonen E. Treatment of infantile spasms: results of a population-based study with vigabatrin as the first drug for spasms. Epilepsia. 1999. 40:950–957.
33. Koo B. Vigabatrin in the treatment of infantile spasms. Pediatr Neurol. 1999. 20:106–110.
34. Chiron C, Dumas C, Jambaque I, Mumford J, Dulac O. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res. 1997. 26:389–395.
35. Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999. 14:71–74.
36. Vanhatalo S, Paakkonen L. Visual field constriction in children treated with vigabatrin. Neurology. 1999. 52:1713–1714.
37. Veggiotti P, Cieuta C, Rex E, Dulac O. Lamotrigine in infantile spasms. Lancet. 1994. 344:1375–1376.
38. Cilio MR, Kartashov AI, Vigevano F. The long-term use of felbamate in children with severe refractory epilepsy. Epilepsy Res. 2001. 47:1–7.
39. Lawlor KM, Devlin AM. Levetiracetam in the treatment of infantile spasms. Eur J Paediatr Neurol. 2005. 9:19–22.
40. Yanai S, Hanai T, Narazaki O. Treatment of infantile spasms with zonisamide. Brain Dev. 1999. 21:157–161.
41. Mikati MA, Fayad M, Koleilat M, Mounla N, Hussein R, Kazma A, et al. Efficacy, tolerability, and kinetics of lamotrigine in infants. J Pediatr. 2002. 141:31–35.
42. Pellock JM. Managing pediatric epilepsy syndromes with new antiepileptic drugs. Pediatrics. 1999. 104:1106–1116.
43. Cianchetti C, Pruna D, Coppola G, Pascotto A. Low-dose lamotrigine in West syndrome. Epilepsy Res. 2002. 51:199–200.
44. Bourgeois BF. Felbamate. Semin Pediatr Neurol. 1997. 4:3–8.
45. O'Neil MG, Perdun CS, Wilson MB, McGown ST, Patel S. Felbamate-associated fatal acute hepatic necrosis. Neurology. 1996. 46:1457–1459.
46. Suzuki Y, Nagai T, Ono J, Imai K, Otani K, Tagawa T, et al. Zonisamide monotherapy in newly diagnosed infantile spasms. Epilepsia. 1997. 38:1035–1038.
47. Suzuki Y. Zonisamide in West syndrome. Brain Dev. 2001. 23:658–661.
48. Mikaeloff Y, de Saint-Martin A, Mancini J, Peudenier S, Pedespan JM, Vallee L, et al. Topiramate: efficacy and tolerability in children according to epilepsy syndromes. Epilepsy Res. 2003. 53:225–232.
49. Ritter F, Glauser TA, Elterman RD, Wyllie E. Topiramate YP Study Group. Effectiveness, tolerability, and safety of topiramate in children with partial-onset seizures. Epilepsia. 2000. 41:Suppl 1. S82–S85.
50. Albsoul-Younes AM, Salem HA, Ajlouni SF, Al-Safi SA. Topiramate slow dose titration: Improved efficacy and tolerability. Pediatr Neurol. 2004. 31:349–352.
51. Coppola G, Caliendo G, Terracciano MM, Buono S, Pellegrino L, Pascotto A. Topiramate in refractory partial-onset seizures in children, adolescents and young adults: A multicentric open trial. Epilepsy Res. 2001. 43:255–260.
52. Appleton RE. Vigabatrin in the management of generalized seizures in children. Seizure. 1995. 4:45–48.
53. Langtry HD, Gillis JC, Davis R. Topiramte. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in the management of epilepsy. Drugs. 1997. 54:752–773.
54. Levisohn PM. Safety and tolerability of topiramate in children. J Child Neurol. 2000. 15:Suppl 1. S22–S26.
55. Shorvon SD. Safety of topiramate: adverse events and relationships to dosing. Epilepsia. 1996. 37:Suppl 2. S18–S22.
56. Sen HA, O'Halloren HS, Lee WB. Case reports and small case series: topiramte-induced acute myopia and retinal striae. Arch Ophthalmol. 2001. 119:775–777.
57. Ben-Zeev B, Watemberg N, Augarten A, Brand N, Yahav Y, Efrati O, et al. Oligohydrosis and hyperthermia: Pilot study of a novel topiramate adverse effect. J Child Neurol. 2003. 18:254–257.
58. Kossoff EH, Pyzik PL, Furth SL, Hladky HD, Freeman JM, Vining EP. Kidney stones, carbonic anhydrase inhibitors, and the ketogenic diet. Epilepsia. 2002. 43:1168–1171.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download