Abstract
Here we report the case of a 20-year-old female patient previously diagnosed with Hashimoto's thyroiditis and overt hypothyroidism, and who had been taking synthetic thyroxine (100 µg/day) for eight months. She experienced intermittent dizziness and generalized weakness, and was diagnosed as having severe autoimmune hemolytic anemia (AIHA). We prescribed prednisolone treatment and continued synthetic thyroxine administration. Two years and five months later, she developed idiopathic thrombocytopenic purpura (ITP) and was diagnosed with Evans' syndrome. Thereafter, laparoscopic splenectomy was performed because her autoimmune hemolytic anemia was refractory and dependent on steroid therapy. The HLA genotypes of the patient were HLA-A*020101/A*2602, HLA-B*270502/B*5401, HLA-Cw*0102/Cw*020202, HLA-DRB1*0404/DRB1*0405, and HLA-DQB1*0302/DQB1*0401. Hashimoto's thyroiditis is often associated with other nonendocrine autoimmune diseases, and antithyroid antibodies are frequently observed in Evans' syndrome (coexistence of AIHA and ITP). However, there is no report of Evans' syndrome developing in patients with overt hypothyroidism and Hashimoto's thyroiditis. This case suggests that three autoimmune diseases (AIHA, ITP, and Hashimoto's thyroiditis) might share a common immunogenetic pathway in pathogenesis.
Chronic, autoimmune thyroid diseases are sometimes combined with autoimmune hematologic diseases, such as pernicious anemia, autoimmune hemolytic anemia (AIHA) and idiopathic thrombocytopenic purpura (ITP). Hashimoto's thyroiditis is one of the most common autoimmune diseases. It is supported by autoantibody formation and lymphocyte infiltration of the thyroid glands. Since Evans reported the first case of AIHA associated with ITP, there have been several case reports of Evans' syndrome occurring simultaneously with an autoimmune thyroid disease1; nine cases of Evans' syndrome occurring with Graves' disease have been reported,2-10 and one case of Evans' syndrome with Hashimoto's thyroiditis and hypothyroidism has been reported worldwide.11 Here we report a case of Evans' syndrome developed together with Hashimoto's thyroiditis and overt hypothyroidism in a 20-year-old woman.
A 20-year-old female patient, who had taken synthetic thyroxine (100 µg/day) for Hashimoto's thyroiditis and overt hypothyroidism, was admitted to our hospital with symptoms of dizziness and generalized weakness in October 1997. Eight months prior, she had been diagnosed with primary hypothyroidism and had begun treatment with synthetic thyroxine (50 µg/day). Upon admission to our hospital, the patient had aggravated dizziness with accompanying nausea and vomiting. She has five siblings, none of whom has any autoimmune disorder.
On physical examination, the patient's blood pressure was 100/70 mmHg, body temperature, 36.8℃, respiration rate, 20 breaths/minute, and pulse rate, 110/minute. She weighed 51 kg and measured 151 cm height (body mass index 22.4 kg/m2).
She looked pale and acutely ill, and had anemic conjunctiva and icteric sclera. Exophthalmoses were not present. The thyroid gland was slightly enlarged and had a hard consistency on palpation, without tenderness. The abdomen was soft and flat, and the liver and spleen were not palpable.
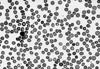
The patient's initial hemoglobin (5.2 g/dL) and hematocrit (15.1%) levels were severely low. Her white blood cell count (WBC) was 9,150/µL (68% polymorphonuclear leukocytes, 18% lymphocytes, 12% monocytes and 2% metamyelocytes), and her platelet count was 576,000/µL. The reticulocyte count was 21.2%, the corrected reticulocyte count was 7.0% and the reticulocyte index was 2.8. Peripheral blood smear analysis revealed spherocytosis, macrocytosis, poikilocytosis, and polychromasia with hemolytic features, and adequate white blood cell and platelet counts (Fig. 1). Levels of both serum calcium and phosphorus were within normal ranges. Total bilirubin, alkaline phosphatase, aspartate transferase and alanine transferase in the serum were 61.2 µmol/dL (reference range [RR], 5.1-17), 87 IU/L (RR, 30-120), 45 and 62 U/L (RR, 5-45), respectively. Lactate dehydrogenase concentration was highly increased to 748 IU/L (RR, 30-170 IU/L), and serum haptoglobin was decreased to 11.8 ng/dL (RR, 50-320 ng/dL). Direct/indirect Coombs' tests and a hemosiderin test yielded strongly positive results. Antinuclear antibody (ANA), antiplatelet antibody, acidified sucrose lysis (Ham's) test and osmotic fragility test were all negative. Abdominal ultrasonography revealed that the patient had a normal sized spleen and liver. According to bone marrow aspiration and biopsy, the patient showed erythroid hyperplasia, which is consistent with hemolytic anemia.
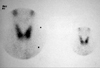
The thyroid function tests performed during synthetic thyroxine (100 µg/day) treatment showed that the patient was at a euthyroid state. However, she had elevated levels of antithyroid antibodies [antithyroglobulin antibody 3,100 U/mL (RR, 0-60) and antimicrosomal antibody 541 U/mL (RR, 0-60)]. The technetium-99m scan (Fig. 2) indicated that she had a slightly enlarged thyroid with an inhomogeneous uptake pattern (2.8%). Based on the above results, the patient was diagnosed as having autoimmune hemolytic anemia and Hashimoto's thyroiditis. Her HLA genotypes, HLA-A*020101/A*2602, HLA-B*270502/B*5401, HLA-Cw*0102/Cw*020202, HLA DRB1*0404/DRB1*0405, and DQB1*0302/DQB1*0401 were determined as previously described.12
We started the patient on an oral administration of prednisolone (1 mg/kg) and continued synthetic thyroxine medication (100 µg/day). Her hemoglobin level increased and stabilized within two weeks after prednisolone treatment. She had regular follow-up with prednisolone (10 to 30 mg/day) and synthetic thyroxine (100 µg/day) for two years and five months. In April 2000, she complained of frequent epistaxis episodes and an increased amount of menstrual bleeding with her regular cycle. She denied ingestion of any drugs including alcohol or herbs during that time period. She had also adhered to the prescribed administration of prednisolone and synthetic thyroxine. Physical examination did not show any clues indicating systemic lupus erythematosus (SLE) such as arthritis, photosensitivity, oral ulcer, skin rash or psychosis. At that time, she had moderate and persisting thrombocytopenia (75,000-90,000/µL), and repeated ANA test results were negative for serum platelet autoantibody. Hepatosplenomegaly was not found by abdominal ultrasonography. Bone marrow biopsy showed normal findings, except a mildly increased number of megakaryocytes. Serum calcium, phosphorus and albumin were 2.3 mmol/L (RR 2.2-2.6), 1.3 mmol/L (RR, 1.0-1.4) and 4.1 g/dL (3.2-5.3), respectively. Her fasting serum glucose level was 4.8 mmol/L. On urinalysis, neither proteinuria nor microscopic hematuria were found.
Considering the occurrence of thrombocytopenia, and regardless of the prednisolone treatment for AIHA and a normal bone marrow biopsy, we concluded that she had refractory idiopathic thrombocytopenic purpura and autoimmune hemolytic anemia, called Evans' syndrome, associated with Hashimoto's thyroiditis. The severity of hemolytic anemia fluctuated with prednisolone dosage, so we were not able to lower her prednisolone dosage below 20 mg/day. In July 2000, the patient had shown aggravated hemolytic anemia, despite a prednisolone dosage of over 30 mg/day. We concluded that the patient was in a steroid dependent and steroid refractory state, and so carried out a laparoscopic splenectomy.
After the operation, the patient's hemoglobin level and platelet count were restored to 11.1 g/dL and 461,000/µL, respectively. We could then lower her prednisolone dosage to 10 mg/day. Currently, she is still taking 10 mg of prednisolone per day due to her steroid-dependent AIHA and ITP condition, and has regular follow-ups at our hospital's outpatient clinic (Table 1).
In a previous study,13 the prevalence of antithyroid antibodies in Evans' syndrome (25%, 5/20) was higher than that in AIHA (11.4%, 5/44) or in ITP (0%, 0/20). In addition, the incidence of subclinical hypothyroidism in Evans' syndrome was 20% (4/20), while it was 20.4% in AIHA (9/44) and 5% in ITP (1/20). Graves' disease was reported as being concomitant with Evans' syndrome in 10% (2/20) of cases, concomitant with AIHA in 0% (0/44) of cases, and with ITP in 5% (1/20) of cases. These findings indicate that tests for abnormal thyroid function and autoimmune thyroiditis are often affected by AIHA, ITP and Evans syndrome. Furthermore, patients with Evans' syndrome more frequently experience autoimmune thyroiditis complications than those with only AIHA or ITP. This suggests that Evans' syndrome might be associated with a specific gene, which may amplify the autoimmune response in the thyroid gland.
There are two distinct types of autoimmune polyendocrine syndromes with characteristic clinical manifestations. APS (autoimmune polyendocrine syndrome) type I, typically recognized in early childhood, is a rare autosomal, recessive disorder. Defects in the autoimmune regulator gene have been shown to cause this disorder.14 In contrast, the more common syndrome, APS type II, typically manifesting in adulthood, has polygenic inheritance. APS type II is less well defined, but is strongly associated with several specific HLA class II gene polymorphisms, frequently HLA DR0301-DQB1*0201, DRB1*0402-, 0404- or 0405-DQB1*0302.15 The chronic development of organ-specific autoimmunity necessitates the evaluation of patients with Evans' syndrome over time. Hashimoto's thyroiditis and Evans' syndrome are autoimmune disorders,16 yet the combined disorders have still not been classified in APS. In this case, HLA DRB1*0404-DQB1*0302 and DRB1*0405-DQB1*0401 haplotypes were common HLA subtypes associated with APS type II. This suggests further that autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura with Hashimoto's thyroiditis might share a common pathogenetic pathway, the defect of which leads to Evans' syndrome and should be classified as an APS type.
In summary, we report a case of Hashimoto's thyroiditis complicated by Evans' syndrome, and suggest that Evans' syndrome could be a high risk factor for autoimmune thyroiditis. Patients with Evans' syndrome should be evaluated by thyroid function tests, including those for thyroid auto-antibodies, to prevent the development of overt hypo- or hyperthyroidism. Although the precise pathogenetic mechanism in Hashimoto's thyroiditis and Evans' syndrome remains to be determined, the two diseases might share a common pathway.
Figures and Tables
Fig. 1
Peripheral blood smear on admission. The peripheral blood smear shows typical hemolytic anemia features such as spherocytosis, poikilocytosis, macrocytosis, and polychromasia of red blood cells, and shows normal findings for white blood cells and platelets (Wright stain, ×400).
References
1. Evans RS, Takahashi K, Duane RT, Payne R, Liu C. Primary thrombocytopenic purpura and acquired hemolytic anemia; evidence for a common etiology. AMA Arch Intern Med. 1951. 87:48–65.
2. Branehög I, Olsson KS, Weinfeld A, Domellöf L. Association of hyperthyroidism with idiopathic thrombocytopenic purpura and haemolytic anaemia. Acta Med Scand. 1979. 205:125–131.
3. Hiraoka N, Setoguchi J, Gotoh I. A case of complication of Evans' syndrome and Basedow's disease. Matsujinkai M J. 1988. 27:79. (in Japanese).
4. Ito M, Ninomiya N, Mizuno H, Ohota H. A case of Evans' syndrome combination of hyperthyroidism. J Jpn Soc Intern Med. 1985. 74:1615. (in Japanese).
5. Sawada Y, Yaombomatani T, Kuroe K, Kanazawa T, Kawazu S. A case of suspicion of Evans' syndrome aggravating anemia and thrombocytopenia associated with hyperthyroid function. Jpn J Clin Haematol. 1989. 30:1642. (in Japanese).
6. Sakai Y, Honda K, Tominaga J, Mitsuwa T, Miyazawa K, Uchida H, et al. A case of Evans' syndrome combination of Graves' disease. Jpn J Clin Endocrinol. 1991. 39:64–66. (in Japanese).
7. Lee FY, Ho CH, Chong LL. Hyperthyroidism and Evans' syndrome. A case report. J Formosan Med Assoc. 1985. 84:256–260.
8. Yashiro M, Nagoshi H, Kasuga Y, Isobe H, Kitajima S, Nakagawa T, et al. Evans' syndrome associated with Graves' disease. Intern Med. 1996. 35:987–990.
9. Ikeda K, Maruyama Y, Yokoyama M, Kato N, Yamanoto H, Kaguchi Y, et al. Association of Graves' disease with Evans' syndrome in a patient with IgA nephropathy. Intern Med. 2001. 40:1004–1010.
10. Geissler D, Ogriseg M, Fill H, Wolf H. Plasmapheresis treatment in 3 simultaneously occurring autoimmune diseases: Hashimoto thyroiditis with hyperthyroidism, autoimmune thrombopenia and autoimmune hemolytic anemia. Wien Klin Wochenschr. 1987. 99:351–355.
11. Hennemann HH, Kloss A. Autoimmune haemolytic anaemia, thrombocytopenia and thyroiditis: an immunopathological triad. Dtsch Med Wochenschr. 1978. 103:609–612.
12. Park MH, Kim HS, Kang SJ. HLA-A, -B, -DRB1 allele and haplotype frequencies in 510 Koreans. Tissue Antigens. 1999. 53:386–390.
13. Lio S, Albin M, Girelli G, Perrone MP, Gandolfo G, Conti L, et al. Abnormal thyroid function test results in patients with Fisher-Evans syndrome. J Endocrinol Invest. 1993. 16:163–167.
14. Aaltonen J, Bjorses P, Perheetupa J, Horelli-kuitunen N, Palotie A, Peltonen L, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997. 17:399–403.
15. Ide A, Eisenbarth GS. Genetic susceptibility in type 1 diabetes and its associated autoimmune disorders. Rev Endocr Metab Disord. 2003. 4:243–253.
16. Kie JH, Cho MS, Yang WI. Expression of CD40 and apoptosis related molecules in autoimmune thyroid diseases. Yonsei Med J. 2001. 42:488–496.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download