Abstract
The effectiveness of percutaneous embolotherapy in cases of hemoptysis due to pulmonary tuberculosis from increasing severity of lung parenchymal injury was compared. The pattern of pleural involvement, as seen on chest radiography and angiography, were comparatively analyzed in 230 patients who were available for follow-ups from March 1992 to December 2003. Chest radiography findings were classified into 4 types based on levels of complicated lesions and pleural involvement. Angiography findings were divided into 4 groups based on the level of blood supply to lesions. Early hemostasis with respect to radiographic group were as follows: Type I- 92% (73/79), Type II- 80% (52/65), Type III- 70% (42/60), and Type IV- 56% (52/92); there was an average success rate of 73% (219/296), and continued hemostasis was found in 80% of Type I patients (62/77), 77% of Type II patients (41/53), 62% of Type III patients (25/40), and 45% of Type IV patients (27/60), with an average long-term hemostatic rate of 67% (155/230). Increasing severity of pleural involvement and associated complications correlated with increasing development of systemic collateral arteries other than the bronchial artery. The severely increased circulation in systemic collateral arteries makes it difficult to predict good hemostatic results following embolization. Therefore, we recommend aggressive treatment, such as surgical intervention, after embolization in such instances.
Pulmonary tuberculosis is the most common cause of hemoptysis, and the onset of this complication may occur after the tuberculosis has been cured or during its inactive stage, with the development of bronchiectasis, chronic bronchitis or aspergilloma, or as the result of the reactivation (recurrence) of tuberculosis, microbial colonization within a bronchial cavitation, scar cancer or complicating hemorrhagic disease.1 Recently, bronchial artery embolizations, as well as other arterial embolizations, have been performed as a preoperative hemostatic measure in the treatment of hemoptysis from a variety of causes, including pulmonary tuberculosis, or as a treatment alternative in cases where surgery is not possible due to poor lung function.2,3 The experience of the present authors revealed that differences exist in both the early and late hemostatic effects of bronchial and systemic collateral artery embolization, depending on whether the patient had tuberculosis without complications, secondary lung lesions due to tuberculosis, or tuberculosis accompanied by pleural involvement. The goal of this study was to investigate the relationship between the degree of lung lesions caused by tuberculosis with the degree of pleural involvement and their influence on the hemostatic effect of arterial embolization.
Among 296 patients who underwent arterial embolization for hemoptysis due to pulmonary tuberculosis from March 1992 to December 2003, 230 were available for follow-up (3-79 months, mean 18.5 months) and were subjects for the present study. There were 143 males and 87 females, and the average age of the subjects was 46 years (range 18-87 years). Plain chest radiography and angiographic studies obtained upon hospitalization were analyzed and interpreted independently by two radiologists retrospectively. The diagnosis of tuberculosis as the cause of hemoptysis was established by either chest radiography (38/230) or chest radiography and sputum examination (192/230). Pre-angiogram bronchoscopy was performed (195/230), as endoscopic findings have been useful in ascertaining the site of bleeding in cases of diffuse lung infiltrations.4
Based on the findings from plain chest radiograph, which included the degree of progression of the tuberculosis, complications, and degree of pleural involvement, the cases were divided into four types Type I was tuberculosis without complicated lesions (Fig. 1), Type II consisted of those cases complicated by conditions such as bronchiectasis, aspergilloma, and cavitation (Fig. 2), Type III consisted of Types I or II in which only the pleura of the lung apices was involved, and Type IV was defined as extensive pleural involvement which exceeded the apices (Fig. 3).
Likewise, angiographic findings were divided into four categories. Group I included cases of bronchial artery abnormalities only. Group II consisted of cases involving either the internal mammary artery or the intercostal artery (Fig. 4A). Group III was defined as either Group I or Group II and an abnormality of one of the branches of the subclavian artery, and Group IV consisted of cases with abnormalities of two or more branches of the subclavian artery, or any case in which there were small branches or blushes that appeared to be collaterals directly from the main trunk (Fig. 4B). The internal mammary artery, a branch of the subclavian artery, was included in Group II because it usually supplies diseased lung parenchyma rather than pleural invasion (Fig. 5). Respective Type and Group data from the patients' charts were compared, and the short- and long-term hemostatic effects of arterial embolization were analyzed.
Images from arterial embolization were captured by DSA with the Optimus DVI System and BV 5000 (Philips, Einthoven, Netherland). Hemorrhages were located with the combination of fiberoptic bronchoscopy, plain chest radiography, and angiographic findings. Any findings of hypervascularity, hypertrophied vessels, bronchial artery-pulmonary artery shunt, arterial aneurysm formation, and extravasation of contrast obtained from angiography were considered as evidence of the bleeding site.
Diagnostic angiography was routinely performed to exclude the presence of spinal arteries arising from the bronchial or intercostal arteries; spinal arteries were not considered a contraindication for embolization. In such cases, a microcatheter catheter tip was positioned distal to the origin of the spinal artery and large particles were used. Embolization was performed with either polyvinyl alcohol (355-500 or 500-710 microns, Contour Emboli, Boston Scientific, USA), gelfoam, or a combination of both. In order to prevent the reflux of the embolization material into the aorta or vertebral artery, the procedure was performed under close fluoroscopic observation with a combination of contrast materials.
Among the 230 patients, 77 had plain chest radiographs classified as Type I, 53 were identified as Type II, 40 were identified as Type III, and 60 had Type IV radiographs. Classification according to abnormal vascular patterns on angiography yielded 101 patients in Group I, 49 in Group II, 35 in Group III, and 45 in Group IV. The interrelationship between the plain chest radiographic findings and abnormal vascular patterns on angiography is presented in Table 1. There was no correlation between age and sex distributions among types of chest radiographs.
The distribution of abnormal angiographic findings according to chest radiographic classification demonstrated an increasing tendency of aneurysm formation, shunt, and extravasation of contrast in Type III and Type IV, providing direct evidence of hemoptysis, in 5 (8%) Type IV patients only, and not in any of the other Types (Table 2).
The distribution and frequency of embolization of the arteries were as follows: bronchial artery, 270 times; intercostal artery, 136 times; internal mammary artery, 71 times; other subclavian branches, 106 times; and inferior phrenic artery, 8 times. The average number of embolized vessels according to Types is as follows; 1.4 of Type I, 1.8 of Type II, 2.7 of Type III, and 4.8 of Type IV. There were more extensive systemic collateral supplies in Type III and IV (11 branches/77 patients in Type I, 14/53 in Type II, 31/40 in Type III, and 121/60 in Type IV) (Table 3). Among 77 patients with failed embolization, 30 underwent operations (2, 10, 13, and 5 in each Type respectively), 25 expired due to hemoptysis during hospitalization (10 of Type III and 15 of Type IV), and 22 were not available for follow up analysis.
There was no re-bleeding after initial arterial embolization in 73 (92%) of Type I, 52 (80%) of Type II, 42 (70%) of Type III, and 52 (56%) of Type IV patients.
These figures represent 219 (75%) out of all 296 patients. Of the 230 patients who were available for long-term follow-up, the number of those who did not experience further episodes of hemoptysis 1 year after treatment (long-term hemostatic rate) was: 62 (80%) of Type I, 41 (77%) of Type II, 25 (62%) of Type III, and 27 (45%) of Type IV patients, which translates to 155/230 (67%) patients overall (Table 4). The number of patients who received surgery as the treatment for re-bleeding was 4/12 (33%) of Type II, 3/15(20%) of Type III, and 2/33 (6%) of Type IV patients; no Type I patients had surgery.
No major complications such as stroke, trans verse myelitis or bronchial infarction were encountered. The anterior spinal artery and the artery of Adamkiewicz were seen in twelve cases (5%), either prior to embolization or only after distal embolization. There were sporadic complaints of chest pain during embolization of hypertrophied intercostal arteries, but they were only transient.
Ever since Remy et al. first performed a bronchial artery embolization to treat hemoptysis in 1974, the procedure has been used to treat both massive hemoptysis and chronic intermittent hemoptysis, as well as a preoperative method to improve the lung function of hemoptysis patients prior to surgery and as an effective hemostatic measure in patients for whom surgery is not possible.5-8
The therapeutic benefits of arterial embolization in hemoptysis vary according to different sources, and depend on a number of factors including the degree of bleeding, risk of recurrent hemoptysis, and the overall lung function of the patient. The severity of hemoptysis cannot be judged by the volume of bleeding alone, as cases with even mild bleeding can be fatal if the bleeding is prolonged for hours or days.1,7
Pulmonary tuberculosis is the major cause of hemoptysis. Both active and inactive tuberculosis can induce hemoptysis. Furthermore, complications due to tuberculosis such as bronchiectasis, aspergilloma, lung cancer, chronic bronchitis, lung abscess, and pneumonia can causes hemoptysis. Effective chemotherapy has simplified the treatment of pulmonary tuberculosis, and a single episode of minor hemoptysis is typically either self-limiting or controllable with antituberculous medication.
Despite recent advances in treatment, pulmonary tuberculosis-induced chronic inflammatory disease continues to be the major cause of hemoptysis, mainly due to the formation of a bronchial-pulmonary artery anastomosis within inflammatory tissue in the vicinity of the bronchioles. Reports show that elevated pressure within the bronchial arteries may result in vascular dilatation or rupture, leading to chronic extravasation of blood, pulmonary cavitation, erosion of the neighboring pulmonary artery, or pulmonary artery aneurysmal rupture due to an arterio-venous fistula.2,3 Therefore, aside from medical therapy based on anti-tuberculous medication alone, aggressive treatment modalities such as bronchial artery embolization or definitive resection of the lesion are necessary.8-10
In this study, the relationship between plain chest radiograph findings and abnormal vascular distribution on angiography of pulmonary tuberculosis patients were compared among four types and groups. We found that when compared to tuberculosis without complications (Type I), tuberculosis accompanied by complications such as bronchiectasis, aspergilloma, and cavitation, etc. (Type II), tuberculosis with limited pleural invasion (Type III) and tuberculosis with widespread pleural involvement (Type IV) all had more involvement of the systemic collateral arteries than that of the bronchial or intercostal arteries; there were more abnormal vessels as well.
An angiographic finding of hypervascularity was evident in all of the cases, and was the most commonly observed indirect sign of hemorrhage. Vascular hypertrophy was present with increasing frequency as the cases progressed from Type I to Type IV. Arterial aneurysm formation was rarely observed in Types I or II, but was rather common in Types III and IV. One of the direct signs of hemoptysis, extravasation of contrast, was evident in only 5 Type IV cases, suggesting that extravasation is primarily observed in cases of widespread pleural involvement of tuberculosis. However, because the interrelationship between extravasation of contrast and findings of plain chest radiographs of pulmonary tuberculosis had not been investigated prior to the present study, we were unable to compare our results with those of others. Arterial embolization in Type I cases was carried out primarily in the bronchial artery, although in some cases it was carried out in the intercostal artery and internal mammary artery. Patients with Type IV chest radiographs had increased systemic collateral vasculature; branches of the subclavian artery were used frequently as the site of embolization in Type IV patients. The parietal pleura is supplied by intercostal, internal mammary, and musculophrenic arteries, whereas the visceral pleura derives its arterial supply from the bronchial arteries. Patients with pleural disease in association with parenchymal abnormalities are more likely to have nonbronchial systemic collateral vessels supplying the bleeding area.11 These findings also suggest that small arteries, which were not initially important, grew into the systemic circulation before embolization supplied a large amount of blood into the lesion, and therefore increased the risk of re-hemorrhage.12 Furthermore, the underlying lesion of pulmonary tuberculosis may progress by a similar mechanism, even after bronchial artery embolization.
There have been many reports on the effectiveness of embolization of hemostasis, and reports on the early hemostatic results include success rates ranging from 73-91%. Continued follow-up after embolization has yielded long-term hemostatic success rates of 72-80% in the literature.9-19 In our study, the short- and long-term hemostatic rates were 73% and 67%, respectively. While these figures are lower overall than previously published results, one should keep in mind that the subjects of this study were patients whose hemoptysis was due to pulmonary tuberculosis only, and included Type IV patients with increased systemic circulation whose early hemostatic rate was 56% and long-term hemostatic rate 45%. Considering that patients in the Type I category, who rarely show signs of systemic collateral circulation, had short- and long-term hemostatic rates of 92% and 80%, respectively, similar to the results of previous literatures, the presence of systemic collateral circulation has an enormous influence on the hemostatic outcome after embolization.17-21
We have limited out discussion to the degree of lung damage due to the cause of the hemoptysis, pulmonary tuberculosis, and the severity of pleural involvement as seen on plain chest radiography, the distribution of abnormal vascularity on angiography, the interrelationship between the latter two findings, and the hemostatic results of embolization. As such, the size and other dimensions of the embolization materials used in this study were not included. We devised a novel classification system for the degree of lung lesion, pleural involvement, and abnormal vascularity on angiography studies. We expect that this information will aid in the prediction of both early and late hemostatic results of arterial embolization for pulmonary tuberculosis patients with hemoptysis, and in the selection of patients who are candidates for aggressive treatment measures such as surgery.
In conclusion, we confirmed that when compared to cases of simple pulmonary tuberculosis or tuberculosis complicated by bronchiectasis, aspergilloma, or cavitation, more systemic collateral circulation existed in cases of tuberculosis accompanied by widespread pleural involvement. Such cases with extensive pleural involvement also had more frequent abnormal vascular findings on angiography, showed direct evidence of hemoptysis such as the extravasation of contrast material, and displayed relatively lower early and long-term hemostatic effects after embolization.
Figures and Tables
Fig. 1
An angiogram shows pulmonary tuberculosis in the right upper lung field (Type I). Right intercostobronchial angiography shows hypervascularity from the bronchial artery (Grade I).
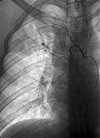
Fig. 3
Destructive change, bronchiectasis, and extensive pleural invasion are seen on a chest PA (Type IV).
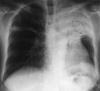
Fig. 4
(A) Hypervascularity and shunting are noted in an angiogram of the right intercostobronchial (Group II). (B) A Right subclavian angiogram shows numerous collateral branches and shunting from the subclavian artery (Group IV).
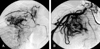
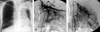
Fig. 5
(A) A Chest PA shows destructive lung parenchyma and pleural invasion in the left upper lung field (Type IV). (B) A left subclavian angiogram shows a hypertrophied internal mammary artery, shunting, and numerous collaterals with blushes from main trunk of the subclavian artery (Group IV). (C) The left internal mammary artery supplies a destructive lung parenchyma rather than the pleural lesion.
ACKNOWLEDGMENTS
We wish to thank Jae Yong Kim, Northfield Mount Hermon School, MA, USA, for his editorial assistance in the preparation of this manuscript.
References
1. Raviglione MC, O'Brien RJ. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson L, editors. Tuberculosis. Harrison's Principles of Internal Medicine. 2005. 16th ed. New York: McGraw-Hill;953–966.
2. Sung YS, Suh KJ, Kim YJ. Bronchial artery embolization: clinical analysis of 129 cases. J Korean Radiol Soc. 1992. 28:505–512.
3. Kim SM, Kim YJ, Yang HS, Lee MS, Sung KJ. Arterial embolization for management of hemoptysis. J Korean Radiol Soc. 1994. 30:1029–1034.
4. Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001. 177:861–867.
5. Remy J, Voisin C, Dupis C, Beguery P, Tonnel AB, Denies JL, et al. Treatment of hemoptysis by embolization of the systemic circulation. Ann Radiol (Paris). 1974. 17:5–16.
6. Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med. 2003. 29:317–320.
7. Remy J, Jardin M. Dondelinger F, Rossi P, Kurdziel JC, Wallace S, editors. Angiographic management of bleeding. Interventional Radiology. 1990. New York: Thieme;325–341.
8. Giron JM, Poey CG, Fajadet PP, Balagner GB, Assoun JA, Richardi GR, et al. Inoperable pulmonary aspergilloma: percutaneous CT-guided injection with glycerin and amphotericin B paste in 15 cases. Radiology. 1993. 188:825–827.
9. Regnard JF, Icard P, Nicolosi M, Spagiarri L, Magdeleinat P, Jauffret B, et al. Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg. 2000. 69:898–903.
10. Picard C, Parrot A, Boussaud V, Lavole A, Saidi F, Mayaud C, et al. Massive hemoptysis due to Rasmussen aneurysm: detection with helicoidal CT angiography and successful coil embolization. Intensive Care Med. 2003. 29:1837–1839.
11. Wong ML, Szkup P, Hopley MJ. Percutaneous embolotherapy for life-threatening hemoptysis. Chest. 2002. 121:95–102.
12. Cho KS, Kim YJ, Kim SM, Sung KJ, Kim DJ, Park JW, et al. Arterial embolization for management of hemoptysis in pulmonary tuberculosis: factors of rebleeding. J Korean Radiol Soc. 1996. 35:183–188.
13. Uflacker R, Kaemmerer A, Picon PD, Rizzon CF, Neves CM, Oliveira ES, et al. Bronchial artery embolization in the management of hemoptysis: technical aspect and long-term results. Radiology. 1985. 157:637–644.
14. Remy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977. 122:33–37.
15. Rabkin JE, Astatfiev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987. 163:361–365.
16. Hayakawa K, Tanaka F, Torizuka T, Mitsumori M, Okuno Y, Matsui A, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992. 15:154–159.
17. Ramakantan R, Bandekar VG, Gandhi M, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996. 200:691–694.
18. Keller FS, Rosch J, Loflin TG, Nath PH, McElvein RB. Nonbronchial systemic collateral arteries: significance in percutaneous embolotherapy for Hemoptysis. Radiology. 1987. 164:687–692.
19. Mal H, Rullon I, Mellot F, Brugiere O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999. 115:996–1001.
20. Yu-Tang Goh P, Lin M, Teo N, En Shen Wong D. Embolization for hemoptysis: a six-year review. Cardiovasc Intervent Radiol. 2002. 25:17–25.
21. Kato A, Kudo S, Matsumoto K, Fukahori T, Shimizu T, Uchino A, et al. Bronchial artery embolization for hemoptysis due to benign disease: immediate and long-term results. Cardiovasc Intervent Radiol. 2000. 23:351–357.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download