Abstract
TNF-related apoptosis inducing ligand (TRAIL) expressions were studied in primary human brain astrocytes in response to pro-inflammatory cytokines. When astrocytes were treated with IL-1β, TNF-α or IFN-γ, TRAIL was induced in cultured fetal astrocytes. In particular, IFN-γ induced the highest levels of TRAIL in cultured astrocytes. When astrocytes were prereated with IFN-γ, they induced apoptosis in TRAIL-sensitive Peer cells. Our results suggest that IFN-γ modulates the expression of TRAIL in astrocytes, which may enhance cytotoxic sensitivity of infiltrating immune cells or brain cells other than astrocytes during inflammation of brain.
In this study brain astrocytes were examined from the standpoint of their cytotoxic effects. During inflammation, significant astrocyte death occurs after a period of reactive gliosis. Such gliosis has been reported in Alzheimer's disease,1 multiple sclerosis2,3 and HIV infections.4 TNF-related apoptosis inducing ligand (TRAIL) is a member of the death ligand/receptor pairs, and acts independently of Fas mediated apoptosis.5 TRAIL is consistently expressed in low-grade astrocytomas, anaplastic astrocytomas, and glioblastomas.6-8 However, little published information is available about the relationship between primary astrocytes and TRAIL, though we previously issued a report which demonstrated that the mRNA of TRAIL is detectable in primary astrocytes after stimulation with pro-inflammatory cytokines.9 When inflammation, tumor progression, or a degenerative disease occurs in the CNS, lymphocytes infiltrate and secrete IFN-γ, priming the immune reactions of astrocytes. We previously reported that the full transcriptional activity of STAT1 is manifested when the Ser727 is phosphorylated, and that this is dependent on p38 MAP kinase in astrocytes.10 Here, the expression of TRAIL in primary astrocytes induced by IFN-γ, and TRAIL-mediated apoptosis were addressed in an attempt to identify the functional role of astrocytes in the apoptosis of brain cells.
Fetal astrocytes were isolated from a 25-week-old human fetus. The brain samples were obtained by therapeutic abortion. The use of tissue samples was approved by the Institutional Review Board. Astrocytes were cultured in 10% FCS (Gibco BRL, Grand Island, NY)-DMEM (Gibco BRL) containing 1% nonessential amino acids (Sigma, St. Louis, MO) at 37℃ in 5% CO2. Primary cultures were maintained for up to ten weeks. Staining for glial fibrillary acidic protein (GFAP) was done using anti-GFAP antibody (Pharmingen, San Diego, CA) to estimate the pure astrocyte population, which revealed approximately 99% of the cells were astrocytes. GFAP is the major component of the intermediate filaments of astrocytes and is commonly used as an astrocyte marker. Staining with anti-HLA-DR antibody (Becton Dickson, Franklin Lakes, NJ) was done to detect microglias and activated astrocytes. Anti-GFAP or anti-HLA-DR antibodies were conjugated with FITC and used as recommended by the manufacture.
For the induction of TRAIL, astrocytes were cultured with IFN-℃ (Genzyme, Cambridge, MA) at a concentration of 250 U/mL or 500 U/mL for 8 or 24 hr. IL-1β (Genzyme) was treated at 100 U/mL, and TNF-α (Genzyme) was treated at 750 U/mL for 8 hr for RNA preparation.
Total RNA was isolated using an RNeasy kit (Qiagen, Santa Claris, CA). The level of TRAIL mRNA was measured by RPA or RT-PCR. For RPA, a RiboQuant multiprobe RNase protection assay kit (Pharmingen) was used with 10 µg of total RNA. For RT-PCR, total RNA (1 µg) was used to synthesize cDNA using 0.1 O.D. of random hexamer (Pharmacia, Uppsala, Sweden) and 200 U of M-MLV reverse transcriptase (Gibco BRL). The TRAIL primers used were; forward: 5'-CCCAATGACGAAGAGAGTATGA-3'; reverse: 5'-GGAATAGATGTAGTAAAACCCT-3', and the PCR conditions were as follows: denaturation at 94℃ for 30 sec, annealing at 59℃ for 30 sec and extension at 72℃ for 30 sec. The PCR buffer contained 10 mM Tris-HCl (pH 10), 2.0 mM MgCl2, 50 mM KCl with 1.25 U of Taq polymerase (Takara, Tokyo, Japan). After 30 PCR cycles, an additional extension at 72℃ for 10 min was performed for all primers. β-actin was used as the internal control. Southern blotting was performed using the TRAIL PCR product (100 ng) labeled using an ECL kit (Amersham life Science, Piscataway, NJ) and hybridized at 42℃ overnight.
To evaluate the function of the induced TRAIL, astrocytes pre-treated with IFN-γ for 24 hr were reacted with Peer cells, which are sensitive to TRAIL-mediated apoptosis.11 Peer cells are of lymphoid origin, and were maintained in suspension culture at 10% FCS-RPMI. Adherent astrocytes (effector cells) and floating Peer cells (target cells) were reacted at E:T ratios of 1:1, 2:1, or 4:1. Reactions were performed in 10% RPMI 1640 for 24 hr. Apoptotic cells were stained using Annexin V-FITC (Trevigen, Gaithersburg, MD) and dead cells using propidium iodide (Sigma, St. Louis, MO). 1 × 105 cells were analyzed using a FACstar (Becton Dickinson, San Jose, CA).
For western blotting, each protein (40 µg) of each whole cell homogenate was loaded on 12% PAGE. Proteins were blotted onto a nitrocellulose membrane (Amersham Pharmacia Biotech., Piscataway, NJ) and incubated with 5 µg/ml of polyclonal mouse anti-human TRAIL antibody (Pharmingen). After three washes in PBS containing 0.1% Tween 20, the membranes were incubated with secondary peroxidase-conjugated goat anti-mouse IgG (Jackson Immuno Research, Baltimore, MD) for 1 hr, washed three times in PBS containing 0.1% Tween 20, and developed using an ECL system (Amersham Pharmacia Biotech).
To evaluate TRAIL expression RPA was done after treating brain astrocytes with IFN-γ, IL-1, or TNF-α. All of these cytokines induced TRAIL expression, but the effect of IFN-γ was prominent (Fig. 1A). RT-PCR and the subsequent southern blot analysis of the RT-PCR product revealed that TRAIL mRNA was present at a very low level (Fig. 1B), and that IFN-γ increased the level of TRAIL RNA. Western blotting showed TRAIL protein was also increased by IFN-γ (Fig. 1C).
To determine the biological function of the expressed TRAIL, primary astrocytes treated with IFN-γ for 24 hr were reacted with Peer cells, which have been reported to be TRAIL-sensitive target cells. After reaction with astrocytes (effector cells), floating Peer cells (target cells) were collected carefully; the astrocytes remained adherent. The Peer cells were then stained with annexin V-FITC and propidium iodide. The percentages of annexin V positive cells in the target Peer cell population were 26.0% (E:T = 1:1), 41.4% (E:T = 2:1) or 43.9% (E:T = 4:1) after reaction with IFN-γ treated astrocytes as compared with 3.9% (E:T = 1:1), 5.3% (E:T = 2:1), or 6.2% (E:T = 4:1) for IFN-γ untreated astrocytes (Fig. 2). These results suggest that induced TRAIL molecules on astrocytes are functionally active and trigger apoptosis in proper target cells. Treatment with IFN-γ for 24 hr did not induce any apoptotic changes in primary astrocytes themselves (data not shown).
Astrocytes have been reported to play a significant role in the immune responses of the CNS.12 They produce various cytokines 13 and express the cell death mediated molecules Fas and FasL.3,9 Here, we report that the expression of TRAIL can be induced in primary astrocytes by IFN-γ treatment. In this study, human astrocytes expressed TRAIL at very low levels during primary culture, however, IL-1β, TNF-α, and more significantly IFN-γ up-regulated TRAIL expression. However, it has been suggested that the human brain lacks TRAIL expression,14 this difference may reflect the in vivo status of TRAIL expression. The low level of TRAIL expression detected in control astrocytes in our study may have been due to the slight activation of astrocytes during culture. However, we observed TRAIL expression after IFN-γ treatment by western blotting. In line with the results of the present study, a recent report showed that TNF and TRAIL do not induce apoptosis in reactive astrocytes.15 IFN-γ has been shown to increase the expression of the TRAIL in human colon cancer cell lines16 and in human monocytes.17 Moreover, the up-regulation of TRAIL expression has been reported in NK cells of the liver, spleen, and lungs, by the administration of IL-12, a powerful inducer of IFN-γ production in NK cells and NKT cells.18 Moreover, TRAIL expression was not detected in the NK cells of IFN-γ knock out mice.18
In conclusion, the following were found in human brain astrocytes; 1) TRAIL was expressed at a very low level and this expression was increased by IFN-γ stimulation, 2) astrocytes can induce TRAIL-mediated apoptosis when they were reacted with appropriate target cells. Moreover, the up-regulation of TRAIL may contribute to immune privilege during fetal brain development and to the apoptosis of neighboring cells during pathological conditions.
Figures and Tables
Fig. 1
Effect of IFN-γ on TRAIL expression in astrocytes. 2 × 105 astrocytes were pre-treated with IFN-γ (250 U/mL) for 8 hr. (A) RPA was performed using 10 µg total RNA. IL-1 (100 U/mL) or TNF-α (750 U/mL) was treated for 8 h. (B) TRAIL expression was determined by semi-quantitative RT-PCR, and PCR product obtained was subjected to southern blotting. β-actin was used as an internal control. RT-PCR was performed in quadruplicate and representative results are shown. (C) For western blotting 40 µg of each cell homogenate were loaded on a 12% PAGE. Proteins were blotted onto a nitrocellulose membrane and incubated with 5 µg/mL of polyclonal mouse anti-human TRAIL antibody.
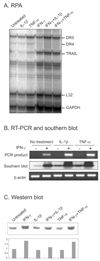
Fig. 2
Induction of apoptosis of Peer cells by astrocytes treated with IFN-γ. 2 × 105 fetal brain astrocytes were pre-treated with 250 U/mL of IFN-γ for 24 hr. Effector cells (astrocytes) and target cells (Peer cells) were cocultured for 24 hr at an E:T ratio of 1:1, 2:1 or 4:1. After collecting floating Peer cells, cell death was measured by staining with annexin V-FITC and propidium iodide. Total 1 × 105 cells were analyzed by a flow cytometry. Astrocytes not treated with IFN-γ were used as control effector cells. The assay was performed in quadruplicate and representative results are shown.

References
1. Bach JH, Chae HS, Rah JC, Lee MW, Park CH, Choi SH, et al. C-terminal fragment of amyloid precursor protein induces astrocytosis. J Neurochem. 2001. 78:109–120.
2. Andersson K, Olofsson A, Nielsen EH, Svehag SE, Lundgren E. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochem Biophys Res Commun. 2002. 294:309–314.
3. Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med. 1996. 184:1513–1518.
4. Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, et al. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996. 98:1979–1990.
5. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995. 3:673–682.
6. Frank S, Kohler U, Schackert G, Schackert HK. Expression of TRAIL and its receptors in human brain tumors. Biochem Biophys Res Commun. 1999. 257:454–459.
7. Nakamura M, Rieger J, Weller M, Kim J, Kleihues P, Ohgaki H. APO2L/TRAIL expression in human brain tumors. Acta Neuropathol (Berl). 2000. 99:1–6.
8. Rieger J, Ohgaki H, Kleihues P, Weller M. Human astrocytic brain tumors express AP02L/TRAIL. Acta Neuropathol (Berl). 1999. 97:1–4.
9. Choi C, Park JY, Lee J, Shin EC, Ahn YS, Kim CH, et al. FasL and Fas are expressed constitutively in human astrocytes and the expression is increased with IL-1, IL-6, TNF-α or IFN-γ. J Immunol. 1999. 162:1889–1895.
10. Lee J, Shin JS, Park JY, Kwon D, Choi SJ, Kim SJ, et al. p38 mitogen-activated protein kinase modulates expression of tumor necrosis factor-related apoptosis-inducing ligand induced by interferon-gamma in fetal brain astrocytes. J Neurosci Res. 2002. 74:884–890.
11. Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999. 163:1906–1913.
12. Eddleston M, Mucke L. Molecular profile of reactive astrocytes: implications for their role in neurologic disease. Neuroscience. 1993. 54:15–36.
13. Aloisi F, Borsellino G, Care A, Testa U, Gallo P, Russo C, et al. Cytokine regulation of astrocyte function: in-vitro studies using cells from the human brain. Int J Dev Neurosci. 1995. 13:265–274.
14. Dorr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH, et al. Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci. 2002. 22:RC209. (1-5).
15. Falsig J, Latta M, Leist M. Defined inflammatory states in astrocyte cultures: correlation with susceptibility towards CD95-driven apoptosis. J Neurochem. 2004. 88:181–193.
16. Langaas V, Shahzidi S, Johnsen JI, Smedsrod B, Sveinbjornsson B. Interferon-gamma modulates TRAIL-mediated apoptosis in human colon carcinoma cells. Anticancer Res. 2001. 21:3733–3738.
17. Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine. TRAIL. J Exp Med. 1999. 189:1343–1354.
18. Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001. 193:661–670.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download