Abstract
We retrospectively reviewed the medical records of 189 children who were admitted to the Pediatric Neurology Department at Yonsei University College of Medicine with status epilepticus (SE) between April, 1994 and April, 2003. The children were followed up for a mean duration of 17 months. We analyzed the clinical findings and the relationships between neurologic sequelae, recurrence, age of onset, presumptive causes, types of seizure, seizure duration and the presence of fever. Mean age at SE onset was 37 months. Incidences by seizure type classification were generalized convulsive SE in 73.5%, and non-convulsive SE in 26.5%. The incidences of presumptive causes of SE were idiopathic 40.7%, epilepsy 29.1%, remote 16.4% and acute symptomatic in 13.3%. Among all the patients, febrile episodes occurred in 35.4%, especially in patients under 3 year old, and 38.4% of these were associated with febrile illness regardless of presumptive cause. Neurologic sequelae occurred in 33% and the mortality rate was 3%. Neurologic sequelae were lower in patients that presented with an idiopathic etiology and higher in generalized convulsive SE patients. The recurrence of SE was higher in patients with a remote symptomatic epileptic etiology, and generalized convulsive SE showed higher rates of recurrence. Based on this retrospective study, the neurologic outcomes and recurrence of SE were found to be strongly associated with etiology and seizure type. Age, seizure duration and the presence of febrile illness were found to have no effect on outcome.
Status epilepticus (SE) is a common neurological emergency that is sometimes associated with high morbidity and mortality. Approximately 10 to 12% of children and adults that experience a first unprovoked seizure1-3 or who are newly diagnosed as having epilepsy1,4 request medical attention for SE. Several clinical series have presented information concerning some important clinical features of SE and predictive indicators of its outcome. Several studies indicate that neurologic outcome seems to be dependent on age and etiology. Neurologic outcome is worse in elderly patients, patients with an acute symptomatic causes or both.5-8 In a study by Maytal et al., neurologic sequelae of convulsive SE were found age dependent, and to occurr in 6% of those over 3 years old but in 29% of patients under 1 year old.9 In this study, we analyzed our data to identify factors predicting the neurologic outcome and recurrence of SE.
The study group included all patients younger than 15 years of age who were admitted with a diagnosis of status epilepticus to the Department of Pediatric Neurology, Yonsei University College of Medicine between April 1995 and April 2003.
The 189 cases were reviewed with respect to seizure history, electrophysiologic data, the immediate precipitating seizure etiology, outcome, laboratory studies and hospital course.
Seizure types and causes were classified according to the International League of AntiEpilepsy classification and guideline for epidemiological research. Status epilepticus was defined as any seizure lasting for 30 minutes or longer or intermittent seizures lasting for more than 30 minutes from which the patient did not regain consciousness. Acute symptomatic cause was defined as any seizure originating from a CNS insult such as anoxia, CVA, hemorrhage, trauma, tumor or a metabolic etiology. A remote symptomatic etiology was defined as one having no acute precipitating etiology, but with a previous history of CNS insult.
An idiopathic etiology was defined as seizures not associated with any identifiable cause of seizure initiation.
We used statistical methods that took into account the time dependent nature of the data. Univariate and multivariate analysis were performed using the Cox proportional hazards models. Risk ratio were used as a measures of the magnitude of the association between variables and the risk of a measured outcome. 95% confidence intervals were calculated using a logistic regression models using a formula based on a normal approximation. The Pearson's chi-square test was also used. A p value of < 0.05 was considered statistically significant.
The SE population was 59% male and 41% female.
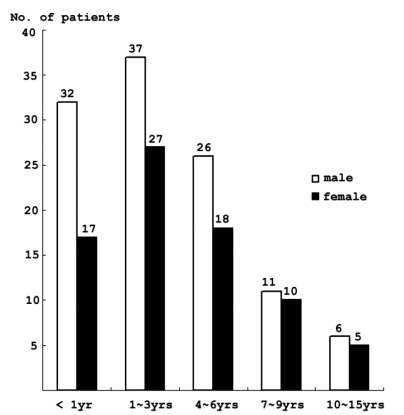
Forty-nine (26%) patients were under the age of 1 year, and 64 patients(34%) were between 1 and 3 years (Fig. 1).
Children less than 3 years of age represented a subgroup of the pediatric population with the highest incidence of SE.
The main etiology in children was idiopathic and accounted of 40.7% of the cases. The epileptic, the remote symptomatic and the acute symptomatic etiology groups also accounted for a significant number of cases in children (Fig. 2). Patients with febrile SE represented 35.4% of all patient regardless of etiology. 38.4% of patients under 3 years of age experienced febrile illness.
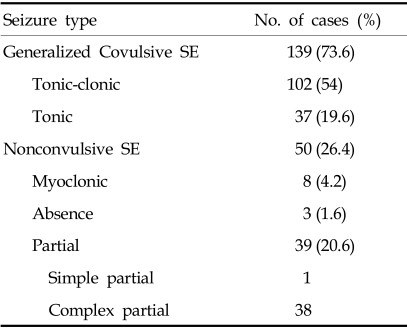
73.6% of SE seizures were generalized convulsive and 26.4% nonconvulsive. Nonconvulsive SE was subdivided into myoclonic, partial and absence SE. Complex partial SE was more common than other nonconvulsive SE. Absence SE was uncommon in this study. Generalized convulsive SE was further divided into generalized tonic-clonic or tonic SE (Table 1).
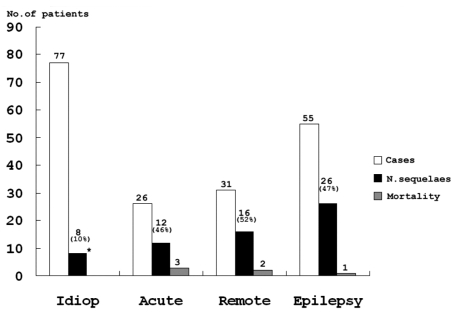
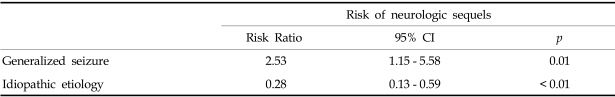
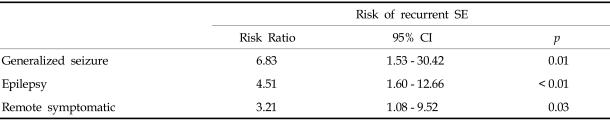
A neurologic sequela was present in 62 cases (33%) (Fig. 3). We analyzed data for factors precicting neurologic outcome i.e., age of onset, presumptive cause, types of seizure, seizure duration and the presence of fever. 79.4% of all SE patients showed a generalized seizure pattern, and generalized seizure was found to be associated with a poor neurologic outcome. SE with an idiopathic etiology showed a better neurologic outcome than the other etiologies (Table 2). Among 55 patients less than 1 year old, 29.1% showed neurologic sequelae. 34.3% of 134 patients over 1 year old showed a poor neurologic outcome, but there wad no significant statistical difference between the two age groups. 70.9% of patients had a short duration of seizure of < 1 hour, and among SE patients, 35.4% of cases had experienced an associated febrile illness, but these two factors did not affect the prevalence of neurologic SE sequelae. Among the 189 patients with SE, 30 (16%) experienced recurrent episodes. The risk factors for a higher rate of recurrent SE are presented in Table 3.
Generalized convulsive seizure, patients with epilepsy and a remote symptomatic etiology showed a higher rate of recurrent SE.
The overall mortality rate among patients group was 3%. The mortality rate was 11.6% among acute symptomatic cases, and 6.5% among remote symptomatic cases. The mortality rate was highest in acute symptomatic cases.
In 1981, the International League against Epilepsy defined status epilepticus as a seizure that 'persist for a sufficient length of time or is repeated frequently enough that recovery between attack does not occur'.10 Some studies have defined status epilepticus as seizures that persist for 20 to 30 minutes,11-13 or as seizures of more than 30 minutes of continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures.11,12 In some other studies, including the VA Cooperative Trial on the Treatment of Generalized Convulsive Status Epilepticus14 and the Pre-Hospital Treatment of Status Epilepticus (PHTSE) study,15 seizure duration of 10 and 5 minutes were used, respectively as SE inclusion criteria. However from the clinical aspect, a definition of a short duration is not widely accepted. So we used the definition that status epilepticus is a seizure lasting over 30 minutes.
DeLorenzo et al. identified patients with prolonged seizures that lasted between 10 and 29 minutes and in the present study, seizures of this duration were quite common, and almost one half would spontaneously terminated without treatment.16
However all investigators agree that the initiation of treatment should begin early to avoid potential neuronal damage secondary to prolonged seizure activity.17 The incidence of SE has been estimated at 10 to 41 cases per 100,000 people per year, totaling some 100,000 to 160,000 patients per year in the United States.18-20 About 10 to 12% of children and adults that experience a first unprovoked seizure1-3 or with newly diagnosed epilepsy1,4 present for medical attention with SE. Approximately 5% of adults and 10% to 25% of children with epilepsy will have at least one episode of SE,21 whereas 13% of all patients with SE will have a recurrent bout.22 The recurrence of SE in the Richmond population was 13.3%18 whereas in our study the recurrence rate was 16%. A remote symptomatic etiology and a young age have long been recognized as risk factors for SE4,11,18,23,24 and recurrent SE.25,26 Shinnar et al. found that 40% of cases among children occurred in those younger than 2 years.23 Moreover, the risk of recurrence is greatest in children with acute and chronic central nervous system insults.25
Our data showed somewhat different features. A remote symptomatic etiology and a previous history of epilepsy were risk factors of recurrent SE, but acute symptomatic etiology did not show significant difference in terms of neurologic outcome. Moreover, age of onset seemed to be unrelated to the rate of recurrence. Fever and infection are the most common causes of generalized convulsive SE in children younger than 2 years of age without a history of epilepsy. However, preexisting epilepsy and low AED levels still remain the primary causes in older children, and appropriate attention to AED levels is warranted.23,25 Remote neurologic insults such as head trauma can also cause SE.3,9
In our study, fever related episodes occurred in 38.4% of children younger than 3 years of age, but this febrile illness did not affect neurologic outcome or SE recurrence.
It is now well and widely accepted that SE can be injurious to the mature brain.
The fundamental pathophysiology of SE involves a failure of mechanisms that normally abort an isolated seizure. This failure can arise from abnormally persistent excessive excitation or the ineffective recruitment of inhibition.27
Induced seizures may be prolonged by a failure of the GABA-mediated suppression of an activated focus. This resistance may develop through the seizure-induced formation of varying isoforms of the GABA type A receptor in the hippocampus.28,29 Alternatively, SE may be perpetuated by sustained N-methyl-D-aspartate (NMDA)-mediated neuronal stimulation.30 In animal models, SE developed a sensitivity to NMDA antagonist in parallel with the development of GABAA resistance.31,32 The presumed mechanism of neurologic injury is via glutamate activation of NMDA receptors leading to programmed cell death.22 The accidental ingestion of mussels contaminated with the neurotoxin domoate, a structural analog of glutamate and kainate, has been reported to cause a profound encephalopathy and prolonged seizures in some patients.33,34
Moreover, SE can be caused by penicillin and related compounds that antagonize the effect of GABA.35 Thirty minutes before, convulsive status epilepticus the glucose and oxygen requirement of the brain are increased. Initially these metabolic demands are met by the dilation of cerebral blood vessels and increases in blood flow.36
But, 30 minutes after seizure, blood pressure and cerebral blood flow reduce and this results in the accumulation of toxic metabolites and inadequate oxygenation.37 Thus SE lasting 30 to 45 minutes can cause cerebral injury, especially to limbic structures like the hippocampus, and the seizure itself is sufficient to damage the central nervous system.38,39
However, the continuing seizure activity also contributes to neuronal damage, independent of systemic factors.40 Such neuronal damage during prolonged SE may be due in part to the action of excitatory neurotransmitters, which cause an influx of calcium into neurons.41
Prolonged seizures even with control of associated systemic disturbances can directly cause cerebral damage.42,43 The morbidity and mortality of SE has been suggested to be related to three major factors44: damage to the CNS caused by the initial insult causing SE, systemic stress from repeated convulsions, and direct injury to the brain due to severe electrical discharges within the central nervous system.
One review of morbidity and mortality rates among children with SE showed neurologic complication rates of 29% among infants younger than 1 year. 11% among children aged 1 to 3 years and 6% among children older than 3 years of age. In 30% of patients, a chronic seizure disorder developed and the mortality rate was approximately 3%.9 Some studies have suggested that the mortality rate among people with SE ranges 6% to 30%.45-47
Etiology has repeatedly been proven to be the major determinant of SE mortality.7,48,49 Although children have a high incidence of SE, there is a much lower overall rate of mortality in the pediatric population than in the adult population.8,18,19,50,51 Permanent neurologic sequelae other than subsequent seizures have been noted in 9% to 28% of children.52 The frequency of neurologic sequelae is highest in children when SE is caused by an acute CNS insult.52 Several studies have shown that the duration of seizure is strongly associated with the ultimate outcome. Moreover, in status epilepticus lasting longer than one hour, outcome is poor.53 The mortality rate increased fivefold in those in the acute symptomatic group with SE lasting over 24 hours.54 Age also seems to be a clear prognostic factor, but when corrected for etiology, it becomes less significant.7
In the present study, the mortality rate was 3%, but among etiologies, an acute symptomatic etiology exhibited the highest mortality. Patients with an idiopathic etiology showed a significantly lower incidence of neurologic sequelae than other causes of SE. Acute symptomatic, remote symptomatic and a previous history of epilepsy show higher rates of neurologic sequelae. However age of onset and duration of seizure were not found to be significantly related with neurologic outcome. It is not clear why age of onset and seizure duration did not affect neurologic outcome. But 71% of patients had a duration of seizure of < 1 hour, perhaps this due to improved management technicques. Based on these results of our retrospective study, we conclude that an idiopathic SE etiology is a favorable predictor of a better neurologic outcome and lower SE recurrence and that generalized convulsive SE an indicator of worse outcomes. However, age of onset and duration of seizure were not found to affect neurologic outcome and SE recurrence.
References
1. Hauser WA. Status epilepticus:epidemiologic considerations. Neurology. 1990; 40(Suppl 2):9–13. PMID: 2185441.
2. Shinnar S, Berg AT, Moshe SL, O'Dell C, Alemany M, Newstein D, et al. The risk of seizure recurrence after a first unprovoked afebrile seizure in childhood: an extended follow-up. Pediatrics. 1996; 98:216–225. PMID: 8692621.

3. Hauser AW, Anderson VE, Loewenson RB. Seizure recurrence after a first unprovoked seizure. N Engl J Med. 1982; 307:522–528. PMID: 7099224.

4. Berg AT, Shinnar S, Levy SR, Testa FM. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol. 1999; 45:618–623. PMID: 10319884.

5. Cascino GD, Hesdorffer D, Logroscino G, Hauser WA. Morbidity of nonfebrile status epilepticus in Rochester, Minnesota, 1965-1984. Epilepsia. 1997; 39:829–832. PMID: 9701372.

6. Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994; 35:27–34. PMID: 8112254.

7. Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997; 38:1344–1349. PMID: 9578531.

8. DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995; 12:316–325. PMID: 7560020.

9. Maytal J, Shinnar S, Mosche SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989; 83:323–331. PMID: 2919138.

10. Proposal for revised clinical and electrophysiologic classification of epileptic seizure: from the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981; 22:489–501. PMID: 6790275.
11. Dodson WE, DeLorenzo RJ, Pedley TA, Shinnar S, Treiman DM, Wannamaker BB. Treatment of convulsive status epilepticus: Recommendation of the Epilepsy Foundation of America Working Group on Status Epilepticus. JAMA. 1993; 270:854–859. PMID: 8340986.
12. Shorvon S. Status epilepticus : its clinical features and treatment in children and adults. 1994. Cambridge, England: Cambridge University Press.
13. Bleck TP. Convulsive disorders:Status epilepticus. Clin Neuropharmacol. 1991; 14:191–198. PMID: 2070361.
14. Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med. 1998; 339:792. PMID: 9738086.

15. Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999; 40:120–122. PMID: 9924914.

16. DeLorenzo RJ, Garnett LK, Towne AR, Waterhouse EJ, Boggs JG, Morton L, et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999; 40:164–169. PMID: 9952262.

17. Treiman DM. Laidlaw J, Richens A, Chadwick D, editors. Status epilepticus. A Textbook of Epilepsy. 1993. 4th ed. Edinburgh, Scotland: Churchill Livingstone;p. 205–220.

18. DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996; 46:1029–1035. PMID: 8780085.

19. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998; 50:735–741. PMID: 9521266.

20. Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: EPISTAR. Neurology. 2000; 55:693–697. PMID: 10980736.

21. Shorvon S. The management of status epilepticus. J Neurol Neurosurg Psychiatry. 2001; 70(Suupl 2):II22–II27. PMID: 11385046.

22. Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000; 41(Suppl 2):S23–S30. PMID: 10885737.

23. Shinnar S, Pellock JM, Moshe SL, Maytal J, O'Dell C, Driscoll SM, et al. In whom does status epilepticus occur. Age related difference in children. Epilepsia. 1997; 38:907–914. PMID: 9579892.
24. Hauser WA. Delgado-Escueta AV, Wasterlain CG, Treiman DM, editors. Status epilepticus, frequency, etiology and neurological sequelae. Status epilepticus, advance in neurology. 1983. 34. New York: Raven Press;p. 3–14.
25. Shinnar S, Maytal J, Krasnoff L, Mosche SL. Recurrent status epilepticus in children. Ann Neurol. 1992; 31:598–604. PMID: 1514772.

26. Driscoll SM, Towne AR, Pellock JM, DeLorenzo RJ. Recurrent status epilepticus in children(Abstract). Neurology. 1990; 40(Suppl 1):297.
28. Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the efficacy of antiepileptic drug during the course of self sustaing status epilepticus. Brain Res. 1998; 814:179–185. PMID: 9838100.
29. Macdonald RL, Kapur J. Acute cellular alterations in the hippocampus after status epilepticus. Epilepsia. 1999; 40(Suppl 1):S9–S20. PMID: 10421557.

30. Bleck TP. Refractory status epilepticus in 2001[editorial]. Arch Neurol. 2002; 59:188–189. PMID: 11843688.
31. Walton NY, Treiman DM. Motor and electroencephalographic response of refractory experimental status epilepticus in rats to treatment with MK-801, diazepam or MK-801 plus diazepam. Brain Res. 1991; 553:97–104. PMID: 1933279.

32. Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res. 2000; 42:117–122. PMID: 11074184.

33. Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990; 322:1775–1780. PMID: 1971709.

34. Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, et al. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990; 322:1781–1787. PMID: 1971710.

35. Lothman EW, Bertram EH III, Stringer JL. Functional anatomy of hippocampal seizure. Prog Neurobiol. 1991; 37:1–82. PMID: 1947175.
36. Maytal J. The management of status epilepticus in children. Child Hosp Q. 1991; 3:255–263.
37. Jagoda A, Riggio S. Refractory status epilepticus in adults. Ann Emerg Med. 1993; 22:1337–1348. PMID: 8333641.

38. Corsellis JAN, Bruton CJ. Delagado-Escueta AV, Wasterlain CG, Treiman DM, Porter RJ, editors. Neuropathology of status epilepticus in human. Advance in neurology. Vol 34. status epilepticus: mechanisms of brain damage and treatment. 1983. NewYork: Raven Press;p. 129–139.
39. Sloviter RS. "Epileptic" brain damage in rats induced by sustained electrical stimulation of the perfortant path. I. Acutr electrophysiological and light microscopic studies. Brain Res Bull. 1983; 10:675–697. PMID: 6871737.
40. Roberts MR, Eng-Bourquin J. Status epilepticus in children. Emerg Med Clin North Am. 1995; 13:489–507. PMID: 7737031.

41. Treiman DM. Generalized convulsive status epilepticus in adult. Epilepsia. 1993; 34(Suppl 1):S29–S41. PMID: 7681771.
42. Wasterlain CG. Mortality and morbidity from serial seizures; an experimental study. Epilepsia. 1974; 15:155–176. PMID: 4525503.
43. Nevander G, Ingvar M, Auer R, Siesjo BK. Status epilepticus in well-oxygenated rats causes neuronal necrosis. Ann Neurol. 1985; 18:281–290. PMID: 4051457.

44. Leppik IE. Status epilepticus: the next decade. Neurology. 1990; 40(Suppl 2):4–9. PMID: 2185439.
45. Celesia GG. Prognosis in convulsive status epilepticus. Adv Neurol. 1983; 34:55. PMID: 6829360.
46. Yager JY, Cheang M, Seshia SS. Status epilepticus in children. Can J Neurol Sci. 1988; 15:402. PMID: 3208225.

47. Koul RL, Raj Aithala G, Chacko A, Joshi R, Seif Elbualy M. Continuous midazolam infusion as treatment of status epilepticus. Arch Dis Child. 1997; 76:445–448. PMID: 9196363.

48. Aminoff MJ, Simon RP. Status epilepticus: cause,clinical features and consequences in 98 patients. Am J Med. 1980; 69:657–666. PMID: 7435509.
49. Oxybury JM, Whitty CWM, Logroscino G, Hauser WA. Treatment of nonfebrile status epilepticus in Rochester, Minn, from 1965 through 1984. Mayo Clin Proc. 2001; 76:39–44. PMID: 11155411.
50. Lacroix J, Deal C, Gauthier M, Rousseau E, Farrel CA. Admissions to a pediatric intensive care unit for status epilepticus: a 10-year experience. Crit Care Med. 1994; 22:827–832. PMID: 8181292.
51. Verity CM, Ross EM, Golding J. Outcome of childhood status epilepticus and lengthy febrile convulsion: findings of national cohort study. BMJ. 1993; 307:225–228. PMID: 8369681.
52. Gross-Tsyr V, Shinnar S. Convulsive status epilepticus in children. Epilepsia. 1993; 34(suppl 1):S12–S20.

53. DeLorenzo RJ, Towne AR, Pellock JM, Ko D. Status epilepticus in children,adults, and the elderly. Epilepsia. 1992; 33(Suppl 4):S15–S25. PMID: 1425490.
54. Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Haser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002; 58:537–541. PMID: 11865129.

Fig. 2
Etiologies of SE in children. Etiologies included Idiopathic, Acute symptomatic (CNS infection, Metabolic, Anoxia, ICH, Trauma), Remote (Remote symptomatic), and Epilepsy. Data are expressed as the numbers and percentages of cases with each etiology.
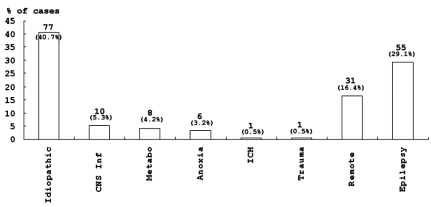
Fig. 3
Neurologic outcomes according to etiologies. Etiologies included Idiop (Idiopathic), Acute symptomatic, Remote symptomatic and Epilepsy. Neurologic sequelae including motor and cognitive deficits and significantly lower in those with an idiopathic etiology than in those with other causes (*p < 0.01).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download