Abstract
Warty squamous cell carcinoma (WSCC), a rare variant of squamous cell carcinoma occurring in younger women, is primarily associated with human papillomavirus (HPV) infection. Although WSCC appears to exhibit less aggressive behavior than typical well-differentiated squamous cell carcinoma, it bears the risk of regional metastasis. Accordingly, WSCC should be differentiated from other verruciform neoplasms. We describe a rare case of WSCC with a short disease duration occurring in a woman of old age. We found the presence of HPV DNA different from other well-known types of high risk and low risk HPV by DNA chip microarray. These results suggest that various types of HPV can be associated with the pathogenesis of WSCC.
Invasive squamous cell carcinoma (ISCC) of the vulva is an uncommon disease, accounting for 3-5% of female genital tract cancers.1 Among them, the warty squamous cell carcinoma (WSCC) is a rarely described lesion that can often be confused with other verruciform tumors, such as condyloma acuminatum and verrucous carcinoma.2,3 Because of the regional metastasis risk of WSCC, it should be differentiated from other verruciform neoplasms based on its histologic findings. This tumor has been associated with a history of vulvar intraepithelial neoplasia, young age, and the presence of human papillomavirus (HPV) DNA.4-6 Here, we report a case of WSCC found in the vulva of a 71-year-old woman and describe its association with HPV using a DNA microarray.
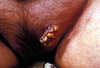
A 71-year-old woman presented with a 6-month history of an enlarging mass of the vulva. Physical examination revealed a 2.5 cm pedunculated, cauliflower-like mass with a verrucous surface on the left side of the vulva (Fig. 1). No regional lymphadenopathy was present. There was no history of any gynecological disease, including vulvar or cervical intraepithelial neoplasia.
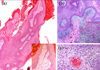
We performed an excisional biopsy. The biopsy specimen revealed a papillomatous pattern with hyperkeratosis, parakeratosis and acanthosis. The dermal papillae had round or tapering, pointed ("spiked") tips, and a central thin fibrovascular core (Fig. 2a). At the deep margin, the tumor invaded the adjacent tissue in the form of irregularly shaped, jagged nests of epithelium. The dermis showed a marked inflammatory reaction (Fig. 2b). Cells within the nests showed mitotic figures, and their nuclei were large, wrinkled, hyperchromatic, with frequent binucleation and multinucleation (Fig. 2c). The lateral margin of the resected specimen showed focal extension of the carcinoma.
We consulted with the gynecology department. However, the patient wanted to go to another hospital for more evaluation. Consequently, no further evaluation and treatment were performed.
For detection of the HPV DNA, a DNA microarray was performed. The HPV DNA chip is an oligonucleotide chip that enables the rapid and easy detection and genotyping of 18 high risk HPV types (i.e., 16/ 18/ 26/ 31/ 33/ 35/ 39/ 45/ 51/ 52/ 56/ 58/ 59/ 66/ 68/ 69/ 70/ 71), 14 low risk HPV types (i.e, 6/ 11/ 32/ 34/ 40/ 42/ 43/ 44/ 53/ 54/ 55/ 57/ 61/ 62) and other types HPV DNA (i.e., positive result in any HPV types, but type is not specified). For HPV genotyping, a commercially available HPV DNA chip was purchased from Biomedlab Co. (Seoul, Korea). The HPV DNA chip contains 32 type-specific probes. The manufacturer's protocol describes the preparation and testing of specimens, and the genotyping experiment was performed using a procedure provided by Biomedlab. Briefly, target HPV DNA was amplified by the polymerase chain reaction (PCR) using the primers (HPV and β-globin) and conditions provided by Biomedlab and labeled using Cy5-dUTP (NEN Life Science Products, Inc., Boston, MA, USA). The PCR product was hybridized onto the chip at 40℃ for 2 hours and washed with 3 × SSPE and with 1 × SSPE for 2 minutes each. Hybridized signals were visualized with a DNA Chip Scanner (GSI Lumonics, Scanarray lite, Ottawa, Canada).
In our case, the DNA microarray showed the existence of other types of HPV DNA, but not the 18 high risk and 14 low risk HPV types (Fig. 3).
WSCC is a rare specific variant of invasive squamous cell carcinoma, usually described as a hybrid feature of condyloma and invasive squamous cell carcinoma. It has been described in the vulva, uterine cervix, penis, anus, oral mucosa and urinary bladder.2,3,6-11 WSCC typically occurs in younger women.1 However, our case developed in old age. The gross appearance of WSCC resembles verrucous carcinoma, being large and exophytic with a papillary appearance, but the surface has a characteristic feathery appearance.7,8 Histologically, the tumors are mainly papillomatous with acanthosis and hyperkeratosis, and they have characteristic histologic findings that include prominent fibrovascular cores of dermal papilla, striking nuclear koilocytotic atypia and jagged, irregular interface between tumor and stroma.8,10
WSCC is a slow growing tumor. However, unlike giant condyloma or verrucous carcinoma, warty carcinomas have a risk of regional metastasis. As such, it must be differentiated from other verruciform neoplasms, such as giant condyloma acuminatum and verrucous carcinoma. Microscopic finding is helpful in differentiating warty carcinoma from giant condyloma. The latter exhibit a pale pink hyperplastic epidermis with uniform-appearing nuclei and much less atypia.12 On the other hand, the former has more cytologic variability, with clear nuclear pleomorphism. In addition, the interface between the base of the process and the underlying tissue is variable and irregular, a feature that is much less evident in condylomatous lesions. WSCC is distinguished from verrucous carcinoma on the basis of long and undulating, condylomatous papilla, with prominent fibrovascular cores and a base that is rounded or irregular and jagged. Furthermore, koilocytotic atypia is prominent and diffuse, while it is absent in the "pure" verrucous carcinoma.7,8,13
HPV infection is strongly associated with warty and basaloid squamous cell carcinoma.14 Several HPV types (e.g., 6, 11, 16, 18, 33) have been detected in the vast majority of WSCC cases.15-17 In the study by Hording et al.,18 84% of basaloid and warty carcinomas were found to harbor HPV type 16 or 33, high risk types, by PCR, while in the same study only 4% of the cases of keratinizing ISCC, which typically develops in older women, had evidence of HPV. In our case, the existence of HPV DNA was proven by DNA chip microarray, but we could not detect the specific HPV DNA type that is often associated with WSCC. Whang et al.19 evaluated the clinical efficacy of the DNA microarray for the detection of HPVs in various cervical lesions. They detected HPV DNAs in 158 and 174 of the 234 cervical samples by PCR-RFLP (restriction fragment length polymorphism) and HPV microarray, respectively, and suggested that HPV oligonucleotide microarray is a highly comparable method to the previously used PCR-RFLP method for the detection of HPV.
In conclusion, we describe here vulvar WSCC occurring in a woman of old age that is associated with an unknown HPV type. This suggests that WSCC may occur regardless of age and that various types of HPV can be associated with the pathogenesis of WSCC.
Figures and Tables
Fig. 2
(a) A papillomatous pattern with hyperkeratosis, parakeratosis and acanthosis (haematoxylin and eosin; original magnification: × 40). Round and tapering dermal papillae with a fibrovascular core (×100). (b) Invasion of the dermis by irregularly shaped, jagged nests of epithelium, the cells which are predominantly mature squamous cells showing atypicality (haematoxylin and eosin; original magnification: ×50). The invading tumor masses are composed of atypical squamous cells (× 200). (c) Cells within the tumor nest demonstrate koilocytosis with large, wrinkled, hyperchromatic nuclei, and horn pearl is present (haematoxylin and eosin; original magnification: ×400).
References
1. Al-Ghamdi A, Freedman D, Miller D, Poh C, Rosin M, Zhang L, et al. Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol. 2002. 84:94–101.
2. Rastkar G, Okagaki T, Twiggs LB, Clark BA. Early invasive and in situ warty carcinoma of the vulva: clinical, histologic, and electron microscopic study with particular reference to viral association. Am J Obstet Gynecol. 1982. 143:814–820.
3. Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993. 17:133–145.
4. Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997. 90:448–452.
5. Trimble CL, Hildesheim A, Brinton LA, Shah KV, Kurman RJ. Heterogeneous etiology of squamous carcinoma of the vulva. Obstet Gynecol. 1996. 87:59–64.
6. Ng WK, Cheung LK, Li AS. Warty (condylomatous) carcinoma of the cervix. A review of 3 cases with emphasis on thin-layer cytology and molecular analysis for HPV. Acta Cytol. 2003. 47:159–166.
7. Bezerra AL, Lopes A, Landman G, Alencar GN, Torloni H, Villa LL. Clinicopathologic features and human papillomavirus and prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol. 2001. 25:673–678.
8. Cubilla AL, Velazques EF, Reuter VE, Oliva E, Mihm MC Jr, Young RH. Warty (condylomatous) squamous cell carcinoma of the penis: a report of 11 cases and proposed classification of 'verruciform' penile tumors. Am J Surg Pathol. 2000. 24:505–512.
9. Noel JC, Sornin de Leysat C, Peny MO, van de Stadt J, Fayt I, De Dobbeleer G. Warty carcinoma of the anus: a variant of squamous cell carcinoma associated with anal intraepithelial neoplasia and human papillomavirus infection. Dermatology. 2001. 203:262–264.
10. Piattelli A, Rubini C, Fioroni M, Iezzi T. Warty carcinoma of the oral mucosa in an HIV+ patient. Oral Oncol. 2001. 37:665–667.
11. Koss LG. Warty carcinoma of bladder containing HPV type 11. Int J Surg Pathol. 2000. 8:367.
12. Brown TJ, Yen-Moore A, Tyring SK. An overview of sexually transmitted diseases. J Am Acad Dermatol. 1999. 41:661–677.
13. Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995. 32:1–21.
14. Kim KH, Kim YS. Role of human papillomavirus and p53 tumor suppressor gene in cervical carcinogenesis. Yonsei Med J. 1995. 36:412–425.
15. Ostrow RS, Shaver MK, Turnquist S, Viksnins A, Bender M, Vance C, et al. Human papillomavirus-16 DNA in a cutaneous invasive cancer. Arch Dermatol. 1989. 125:666–669.
16. Petry KU, Kochel H, Bode U, Schedel I, Niesert S, Glaubitz M, et al. Human papillomavirus is associated with the frequent detection of warty and basaloid high-grade neoplasia of the vulva and cervical neoplasia among immunocompromised women. Gynecol Oncol. 1996. 60:30–34.
17. Cho NH, Joo HJ, Ahn HJ, Jung WH, Lee KG. Detection of human papillomavirus in warty carcinoma of the uterine cervix: comparison of immunohistochemistry, in situ hybridization and in situ polymerase chain reaction methods. Pathol Res Pract. 1998. 194:713–720.
18. Hording U, Daugaard S, Junge J, Lundvall F. Human papillomaviruses and multifocal genital neoplasia. Int J Gynecol Pathol. 1996. 15:230–234.
19. Hwang TS, Jeong JK, Park M, Han HS, Choi HK, Park TS. Detection and typing of HPV genotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol Oncol. 2003. 90:51–56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download