Abstract
Sclerosing encapsulating peritonitis (SEP) is a rare but serious complication in patients with continuous ambulatory peritoneal dialysis (CAPD), and is characterized by a progressive, intra-abdominal, inflammatory process resulting in the formation of sheets of new fibrous tissue, which cover, bind, and constrict the viscera, thereby compromising the motility of the bowel. No satisfactory estimate is available on the comparative incidence of dialysis related SEP and the pathogenesis of SEP still remains uncertain. Although recent therapeutic approaches have reported varying degrees of success, an efficient measure to detect, at an early stage, patients at risk for SEP would be beneficial and a standardized treatment regimen to prevent the illness is urgently needed. This study aimed to evaluate the clinical features of SEP and to identify the possible risk factors for the development of SEP in CAPD patients. We retrospectively reviewed by questionnaire SEP cases among CAPD patients from 7 university hospital dialysis centers in Korea, including Yonsei University, Ajou University, Catholic University, Inha University, Kyungpook University, Seoul National University and Soonchunhyang University, from January 1981 to December 2002. Out of a total of 4,290 CAPD patients in these centers, 34 cases developed SEP with an overall prevalence of 0.79%. The male to female ratio was 17:17. The median age of these patients was 44.5 years (range 19 - 66). The median duration of CAPD before SEP was 64 months (9 - 144) and 68% of patients (23/34) had been on CAPD for more than 4 years. Peritonitis (including two fungal cases) was the main cause of catheter removal in SEP (27 cases, 79%). Seventy-five percent of the cases (15/20) were administered β-blocker for a mean duration of 85 months (26 - 130). Among 10 cases with available peritoneal equilibration test (PET) data, 8 showed high transporter characteristics, and the remaining 2 were high average. Eighteen cases were diagnosed by clinical and radiologic methods, and 16 were surgically diagnosed. Eleven cases were surgically treated and the others were treated conservatively with intermittent total parenteral nutrition (TPN). The overall mortality rate was 24%. SEP is a serious, life threatening complication of CAPD. Most cases had a PD duration of more than 4 years, a history of severe peritonitis, and high transporter characteristics in PET. Therefore, to reduce the incidence of SEP, careful monitoring and treatment, including early catheter removal in patients with severe peritonitis, should be considered for long-term CAPD patients with the above characteristics.
Sclerosing encapsulating peritonitis (SEP) was first described by Gandhi et al.,1 and is a rare but serious complication of peritoneal dialysis (PD), which remains a life threatening condition in PD patients. Due to the rarity and the relatively long development period, there is no satisfactory estimate of the comparative incidence of dialysis related SEP.2 Early diagnosis and treatment prior to the development of symptoms is difficult. Although it is important to detect patients with an increased risk of developing SEP, there is no known clinical predictor for SEP.3,4 Clinical features are variable and include, abdominal pain, nausea, vomiting, weight loss, loss of ultrafiltration, ascites, and blood stained dialysate.5 The diagnosis of disease is usually made at laparotomy, but recently findings on CT scans and ultrasounds of the peritoneum have been used to support diagnosis.6
The purpose of this study was to evaluate the clinical characteristics of SEP patients and to identify clinical features associated with SEP in continuous ambulatory peritoneal dialysis (CAPD) patients. The peritoneal equilibration test (PET), which may herald the occurrence of SEP was conducted on these patients, and some clinical parameters suspected to be related with SEP were determined.
This study was a retrospective multi-center study that reviewed SEP patients in 7 university hospital dialysis centers in Korea.
A questionnaire review was conducted on 4,290 CAPD patients from 7 University hospital dialysis centers in Korea, including Yonsei University, Ajou University, Catholic University, Inha University, Kyungpook University, Seoul National University, and Soonchunhyang University, from January 1981 to December 2002.
Patients were included if the following two criteria were satisfied; Firstly, surgical or radiologic evidence of SEP, such as, dilated fixed loops matted together and tethered posteriorly, adherent dilated bowel loops, loculated ascites, or small-bowel obstruction with thickened peritoneum in the absence of other causes of bowel obstruction. Secondly, CAPD duration was greater than 3 months before the onset of SEP.
Clinical data on SEP patients was obtained retrospectively by chart review in each hospital. Information was obtained regarding the number of cases, age, gender, duration of continuous peritoneal dialysis prior to diagnosis (< 3 month interruption), underlying causes of end-stage renal disease (ESRD), beta blocker usage, interval between PD catheter removal and the diagnosis of SEP and causes of PD catheter removal.
Clinical features of SEP, such as, the number of events of peritonitis and the cumulative duration of peritonitis treatment (total duration of antibiotics usage) before the diagnosis of SEP, causative organisms of peritonitis, ascites after PD catheter removal, nutritional status, and peritoneal transport characteristics were also analyzed. Nutritional status was classified into three groups according to mean serum albumin level over the first 3 months after the diagnosis of SEP (Good: albumin ≥3.0g/dL, Intermediate: 3.0g/dL ≤albumin < 3.5g/dL, Poor: albumin < 3.0g/dL).
Finally, treatment and outcome were evaluated. Treatment duration of SEP was defined as the total reported treatment time (Hemodialysis, total parenteral nutrition, steroid) until a patient became symptom free or expired.
In patients who were given PET, CAPD was performed using commercially available dialysis solution, which contained lactate as a buffer. The last PET results before the onset of SEP were analyzed in this study. Patients used 2-L bags four times a day. The glucose concentration was dependent on the amount of fluid that had been removed.
Peritoneal permeability studies were performed in the absence of peritonitis, using a dialysate volume of 2-L, glucose concentration of 2.5%, and a dwell time of four hours.7,8 After the completion of infusion (0 time) and at 120 minutes dwell time, 200 mL of dialysate was drained. A 10 mL sample was taken and the remaining 190 mL was infused back into the peritoneal cavity. A serum sample was obtained at 120 minutes. At the end of the dwell (240 minutes), the dialysate was drained in the upright position (drain time not to exceed 20 minutes). The drain volume is measured and a 10 mL sample was taken from the drain. All the samples were sent for solute measurement (creatinine, urea, and glucose). Serum and dialysate creatinine concentrations were corrected for a high glucose level, which contributes to non-creatinine chromogens during the creatinine assay. The Dt/D0 glucose and the D/P ratios for creatinine, urea, and others were calculated.
A total of 10 PET available cases were analyzed to investigate peritoneal membrane permeability characteristics.
All values are expressed as median values with a distribution range. Statistical analysis was performed using the statistical package SPSS for Windows Version 11.0 (SPSS, Inc., Chicago, IL, USA). Results were analyzed using the Kruskal-Wallis one-way test and the Mann-Whitney U test. A p-value of less than 0.05 was considered statistically significant.
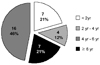
Out of the total of 4,290 CAPD patients admitted between Jan. 1981 and Dec. 2002 in the 7 centers, 34 cases developed SEP with an overall prevalence of 0.79%. The prevalence rates at the individual dialysis centers were 0.58% (12/2,078) in Yonsei for 21 years, 0.28% (2/719) in Kyungpook for 16, 2.86% (14/490) in Soonchunhyang for 9, 0.32% (1/312) in Ajou for 8 years, 1.16% (2/172) in Seoul National for 16, 0.87% (2/230) in Catholic for 7, and 0.35% (1/289) in Inha for 6 (Table 1). Table 2 presents the demographic data of the SEP patients. The male to female ratio was 17:17 and median patient age was 44.5 years (range 19.0 - 66.0). The median duration of CAPD before catheter removal was 64.0 months (range 9.0 - 144.0) and 68% of patients (23/34) had been on CAPD for more than 4 years (Fig. 1). Seventy-five percent of the cases (15/20) used β-blocker for a mean duration of 85.0 months (range 26.0 - 130.0). The median interval between PD catheter removal and a diagnosis of SEP was 4.0 months (range 1.0 - 30.0).
Peritonitis was the main cause of catheter removal in SEP (27 cases, 79%), followed by ultrafiltration failure (5 cases, 15%), and one case each of dialysate leak and exit site infection. The median number of peritonitis events before a diagnosis of SEP was 6 (range 0 - 15) and the cumulative duration of peritonitis was 12.0 weeks (range 0.0 - 84.0). The causative organisms of peritonitis prior to PD catheter removal were methicillin-sensitive Staphylococcus epidermidis (MSSE, 9 cases, 32%), methicillin-sensitive Staphylococcus aureus (MSSA, 7 cases, 25%), Candida species (2 cases, 7%), others (4 cases, 14%), and no growth (6 cases, 21%).
Eighteen cases were diagnosed by clinical and radiologic methods, and 16 cases were surgically diagnosed. The presenting signs and symptoms before a diagnosis of SEP were often gastrointestinal and abdominal pain (10/20, 50%), nausea and vomiting (11/20, 55%) and poor oral intake (4/20, 20%). Thirteen cases showed persistent ascites after catheter removal (37%). Their nutritional statuses after a diagnosis of SEP were poor in 11 cases (32%), intermediate in 15 (44%), and good in 8 (23%). Among 10 cases with available PET data, 8 showed high transporter characteristics and the remaining 2 were high average (Table 3).
Median treatment duration after a diagnosis of SEP was 3.5 months (range 1 - 27). Treatment modalities were hemodialysis only (HD, 16 cases, 46%), HD with total parenteral nutrition (TPN, 6 cases, 17%), HD with surgery (OP, 11 cases, 31%), and HD with steroid (PL, 2 cases, 6%). In surgically treated cases, laparotomies with/without surgical excision of the sclerosed peritoneum were performed. The overall mortality rate was 24%. Eight patients died at an average of 5.3 ± 5.9 months after SEP diagnosis. The mortality showed a higher rate in patients with surgical treatment (4/11, 43%) than in the other treatment groups (Table 4), but this was not statistically significant.
Sclerosing encapsulating peritonitis (SEP) is a rare but serious complication in patients receiving continuous ambulatory peritoneal dialysis (CAPD). SEP, first described by Gandhi et al.1 in 1980, usually occurs in patients who have been on peritoneal dialysis for many years, but sometimes it occurs much earlier.3 The utilization of the peritoneal cavity for dialysis introduces the risk of structural or functional damage to the peritoneal membrane. Small bowel obstruction due to encapsulation, dense adhesions, and mural fibrosis are characteristic and are often associated with peritonitis.2 This disorder is considered to constitute peritoneal sclerosing syndrome with variable clinical features.1-3,9,10
The incidence and prevalence of this syndrome have been defined in some large populations and by a few single-center experiences, but due to the low incidence and the relatively long development, there is no satisfactory estimate of the comparative incidence of dialysis related SEP2. In our study, 34 cases of SEP were diagnosed with a prevalence rate of 0.79%. This prevalence rate is similar to that of Japan, Australia, and Canada with 0.9% (62/6, 923) reported by Nomoto Y et al.,10 0.7% (54/7, 374) by Rigby RJ and Hawley CM,11 and 0.54% (7/1, 288) by Afthentopoulos IE et al.,12 respectively.
The underlying diseases of ESRD in cases with SEP were most commonly chronic glomerulonephritis (48%), followed by hypertension (19%). No patients with diabetes were found even though diabetes mellitus (DM) is the most common cause of ESRD in Korea. This may be partly because technical survival of CAPD patients with DM is much shorter than that of other patient populations. Thereby, they might have been withdrawn from CAPD before the clinical onset of SEP. However, other reasons are also possible.
Although various kinds of causative factors have been reported, there is no known single etiologic factor directly related to SEP.3,4 This is probably because the frequency of the disorder is low and the time needed to develop of clinical disease is protracted.
In terms of the causes of SEP, PD dependent risk factors and PD independent risk factors may be involved,13 such as, PD duration, poor biocompatibility (due to acetate, disinfectant), and peritonitis could be important in the development of SEP,2,5,12,14-19 β-blockers, and a genetic predisposition have also been mentioned as independent factors.20-22
Of the various etiologic risk factors, peritonitis is the most common cause of SEP, and in particular, Staphylococcus aureus, fungi, pseudomonas species, and Hemophilus influenzae are more likely to develop SEP.2,5,11,12,14,16-19,23-26 Loss of mesothelium during peritonitis causes damage to the peritoneal membrane due to bioincompatibilities with substances in PD solutions. Moreover, the loss of fibrinolytic capacity of the peritoneal membrane could contribute to the development of SEP following severe peritonitis.27-29 Antibiotics used in peritonitis, such as, vancomycin and amphotericin B, could also be risk factors of SEP.14,30,31 Peritonitis was the most common cause of PD catheter removal involving 79% of cases in our study. Even though we could not show a direct relationship between peritonitis and the occurrence of SEP, it is likely that peritonitis makes some contribution to its development.
The duration of CAPD is an important determinant factor for SEP. SEP usually occurs in patients receiving CAPD for more than 4 or 5 years, as shown by our data. Rigby RJ and Hawley CM12 in Australia also emphasized the impact of CAPD duration on the development of SEP. They reported a low SEP frequency in patients on peritoneal dialysis for less than 2 years, with a prevalence rate of 1.9%, rising to 6.4, 10.8, and 19.4% in patients on peritoneal dialysis for greater than,5,6 and 8 years, respectively.14 This suggests that nonphysiologic dialysis solutions may induce a chronic sterile inflammation in the peritoneal cavity, thereby inducing several cytokines, which accelerate collagen synthesis by mesothelial cells and fibroblasts. Moreover, the high glucose, high lactate, and low pH in the dialysate can be pernicious to the peritoneal membrane.
Beta-blockers such as practolol or atenolol are known to cause SEP.2,20,21 Practolol is the most commonly implicated β-blocker in the pathogenesis of SEP, and metoprolol, propranolol, and atenolol may also be linked to SEP.20-22 Beta-blockers may inhibit surfactant release and cause damage to various serous membranes in the body.2 We also found that many patients with SEP had a history of β-blocker usage. However, the clinical significance of this drug on the development of SEP was not clarified by in the current study.
Early diagnosis prior to the development of symptoms is difficult6. Patients with SEP can develop gastrointestinal symptoms such as anorexia, nausea, and vomiting due to intestinal obstruction. A loss of dialysis efficiency can also develop. Ascites is an important diagnostic clue to the development of SEP after the suspension of PD.32 In our study, many SEP cases had gastrointestinal symptoms and developed ascites. Symptoms related to SEP are known to be caused by pathologic interference with intestinal function and peritoneal integrity.13 The encapsulating process is responsible for disturbances in intestinal functions that are manifested as disorders of gastrointestinal motility with resultant impairment of reabsorptive disorders, which finally result in protein and calorie malnutrition.
In cases with SEP, an increase in the mass transfer area coefficient of creatinine leads to a progressive loss of ultrafiltration, and decreased CA-125 levels in dialysis fluid were noted due to the loss of mesothelial cells.19,33 Of 10 cases where PET data were analyzed,8 showed high transporter characteristics and the remaining 2 were high averages. The early detection of SEP may be possible if peritoneal solute and fluid kinetics are monitored regularly.
There is no agreement on whether the treatment of choice is surgical, or conservative therapy, consisting of transfer to HD with/without TPN and immunosuppressive drugs including steroids.12 14,30,34-39 Regarding the low frequency of the disease, these treatments have been limited to case reports or small series. The duration of SEP treatment in our study was similar to that of other reports. Twenty-three of 34 patients were treated conservatively and 11 were treated surgically. The mortality of these SEP patients, at 24%, was comparable with that of other reports. The key strategies of conservative treatment are early SEP detection, the cessation of CAPD with transfer to HD, and sustained bowel resting with TPN. Surgical treatment is usually reserved for intestinal obstruction.11,14,16,18,19 Extremely high mortality rates of 60% are due to postoperative complications, typically the opening of an intestinal anastomosis. Our study also showed highest mortality in the surgically treated group, although this was not statistically significant. The reason for this is unclear, but disease severity in this group may have been more severe than in the other groups or the postoperative complications of SEP were frequent, as found by the above studies.
This study has some limitations. First, because it was a retrospective study performed by written questionnaire, the actual prevalence of SEP may have been underestimated because some SEP cases might have been missed due to the unclear diagnostic criteria for SEP. Second, because the number of cases was small, it is difficult to generalize on the characteristics of SEP from the results of our study. Therefore, a larger scale, controlled analysis of the clinical features of SEP is required.
In conclusion, SEP is a rare but serious complication in patients with CAPD. Early PD catheter removal with careful monitoring and treatment should be seriously considered for patients with long-term CAPD exceeding 4 years who experience complications with severe peritonitis, to avoid severe peritoneal damage and the development of SEP.
Figures and Tables
Fig. 1
Distribution of sclerosing encapsulating peritonitis (SEP) cases according to CAPD duration (N=34). SEP was commonly noted in the patients with CAPD duration of more than 4 years.
ACKNOWLEDGEMENT
The authors express their appreciation to staff at the faculties of the six University dialysis centers (Ajou University, Catholic University, Inha University, Kyungpook University, Seoul National University and Soonchunhyang University) for providing the data on the SEP patients.
References
1. Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, et al. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med. 1980. 140:1201–1203.
2. Oules R, Challah S, Brunner FP. Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol Dial Transplant. 1988. 3:66–69.
3. Dobbie JW. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992. 12:14–27.
4. Cox SV, Lai J, Suranyi M, Walker N. Sclerosing peritonitis with gross peritoneal calcification: a case report. Am J Kidney Dis. 1992. 20:637–642.
5. Twardowski ZJ. Clinical value of standardized equilibration tests in CAPD patients. Blood Purif. 1989. 7:95–108.
6. Trivedi H, Khanna R, Lo WK, Prowant BF, Nolph KD. Reproducibility of the peritoneal equilibration test in CAPD patients. Asaio J. 1994. 40:M892–M895.
7. Marichal JF, Faller B, Brignon P, Wagner D, Straub P. Progressive calcifying peritonitis: a new complication of CAPD? Report of two cases. Nephron. 1987. 45:229–232.
8. Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis. 1996. 28:420–427.
9. Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant. 1998. 13:154–159.
10. Afthentopoulos IE, Passadakis P, Oreopoulos DG, Bargman J. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: one center's experience and review of the literature. Adv Ren Replace Ther. 1998. 5:157–167.
11. Garosi G, Di Paolo N. Peritoneal sclerosis: one or two nosological entities? Semin Dial. 2000. 13:297–308.
12. Oreopoulos DG, Khanna R, Wu G. Sclerosing obstructive peritonitis after CAPD. Lancet. 1983. 2:409.
13. Di Paolo N, Garosi G. Peritoneal sclerosis. J Nephrol. 1999. 12:347–361.
14. Holland P. Sclerosing encapsulating peritonitis in chronic ambulatory peritoneal dialysis. Clin Radiol. 1990. 41:19–23.
15. Ing TS, Daugirdas JT, Gandhi VC. Peritoneal sclerosis in peritoneal dialysis patients. Am J Nephrol. 1984. 4:173–176.
16. Slingeneyer A. Preliminary report on a cooperative international study on sclerosing encapsulating peritonitis. Contrib Nephrol. 1987. 57:239–247.
17. Selgas R, Bajo MA, Del Peso G, Jimenez C. Preserving the peritoneal dialysis membrane in long-term peritoneal dialysis patients. Semin Dial. 1995. 8:326–332.
18. Krediet RT. Advances in peritoneal dialysis: towards improved efficacy and safety. Blood Purif. 1998. 16:1–14.
19. Marigold JH, Pounder RE, Pemberton J, Thompson RP. Propranolol, oxprenolol, and sclerosing peritonitis. Br Med J. 1982. 284:870.
20. Myllarniemi H, Leppaniemi A. Peritoneal fibrosis due to practolol. Scanning electron microscopical and histological observations. Acta Chir Scand. 1981. 147:137–142.
21. Nicholls JT, Rutty DA. Sclerosing peritonitis with short-term propranolol therapy. Arch Intern Med. 1980. 140:1124–1125.
22. Eisenberg ES, Alpert BE, Weiss RA, Mittman N, Soeiro R. Rhodotorula rubra peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Am J Med. 1983. 75:349–352.
23. Flanigan M, Anderson D, Freeman RM. Peritoneal dialysis complicated by fungal peritonitis and peritoneal fibrosis. Am J Med. 1984. 76:A113. A125.
24. Tzamaloukas AH. Peritonitis in peritoneal dialysis patients: an overview. Adv Ren Replace Ther. 1996. 3:232–236.
25. Chew CG, Clarkson AR, Faull RJ. Relapsing CAPD peritonitis with rapid peritoneal sclerosis due to Haemophilus influenzae. Nephrol Dial Transplant. 1997. 12:821–822.
26. Verger C, Brunschvicg O, Le Charpentier Y, Lavergne A, Vantelon J. Structural and ultrastructural peritoneal membrane changes and permeability alterations during continuous ambulatory peritoneal dialysis. Proc EDTA. 1981. 18:199–205.
27. Baron MA. Structure of the intestinal peritoneum in man. Am J Anat. 1941. 69:439–496.
28. Jorres A, Gahl GM, Frei U. Peritoneal dialysis fluid biocompatibility: Does it really matter? Kidney Int Suppl. 1994. 48:S79–S86.
29. Junor BJ, McMillan MA. Immunosuppression in sclerosing peritonitis. Adv Perit Dial. 1993. 9:187–189.
30. Charney DI, Gouge SF. Chemical peritonitis secondary to intraperitoneal vancomycin. Am J Kidney Dis. 1991. 17:76–79.
31. Campbell S, Clarke P, Hawley C, Wigan M, Kerlin P, Butler J, et al. Sclerosing peritonitis: identification of diagnostic, clinical, and radiological features. Am J Kidney Dis. 1994. 24:819–825.
32. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000. 20:Suppl 4. S43–S55.
33. Perez-Fontan FJ, Soler R, Sanchez J, Iglesias P, Sanjurjo P, Ruiz J. Retractile mesenteritis involving the colon: barium enema, sonographic, and CT findings. AJR Am J Roentgenol. 1986. 147:937–940.
34. Celicout B, Levard H, Hay J, Msika S, Fingerhut A, Pelissier E. French Associations for Surgical Research. Sclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. Dig Surg. 1998. 15:697–702.
35. Genereau T, Bellin MF, Wechsler B, Le TH, Bellanger J, Grellet J, et al. Demonstration of efficacy of combining corticosteroids and colchicine in two patients with idiopathic sclerosing mesenteritis. Dig Dis Sci. 1996. 41:684–688.
36. Eltringham WK, Espiner HJ, Windsor CW, Griffiths DA, Davies JD, Baddeley H, et al. Sclerosing peritonitis due to practolol: a report on 9 cases and their surgical management. Br J Surg. 1977. 64:229–235.
37. Assalia A, Schein M, Hashmonai M. Problems in the surgical management of sclerosing encapsulating peritonitis. Isr J Med Sci. 1993. 29:686–688.
38. Jackson BT. Surgical treatment of sclerosing peritonitis caused by practolol. Br J Surg. 1977. 64:255–257.
39. Kittur DS, Korpe SW, Raytch RE, Smith GW. Surgical aspects of sclerosing encapsulating peritonitis. Arch Surg. 1990. 125:1626–1628.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download