Abstract
Gastrointestinal system involvement is one of the principal complications seen in the recipients of hematopoietic stem cell transplantation (HSCT), and it is also a major cause of morbidity and death in these patients. The major gastrointestinal complications include typhlitis (neutropenic enterocolitis), pseudomembranous enterocolitis, viral enteritis, graft-versus-host disease, benign pneumatosis intestinalis, intestinal thrombotic microangiopathy, and post-transplantation lymphoproliferative disease. As these patients present with nonspecific abdominal symptoms, evaluation with using such imaging modalities as ultrasonography and CT is essential in order to assess the extent of gastrointestinal involvement and to diagnose these complications. We present here a pictorial review of the imaging features and other factors involved in the diagnosis of these gastrointestinal complications in pediatric HSCT recipients.
Hematopoietic stem cell transplantation (HSCT) is increasingly being used to treat many disorders ranging from hematologic malignancies to solid organ malignancies (1). However, all the recipients are at risk for a variety of post-transplant complications. Gastrointestinal tract involvement is a frequent complication that causes nonspecific symptoms (2, 3). The timing of complications that occur following transplantation is divided into three phases (4). The preengraftment phase lasts 15-30 days and it is characterized by marrow aplasia. The host defense barriers may be weakened during this pancytopenic period. The broadspectrum antimicrobial agents that are administered during this period are also a contributing factor for infection (3). The early post-engraftment period, i.e., from 30 to 100 days after transplantation, is characterized by the restoration of hematopoiesis. However, as lymphocyte recovery occurs more slowly, there is a continued deficiency of both cellular and humoral immunity during this period. The late, post-engraftment period begins only months or years after transplantation (4).
The complications that arise shortly after transplantation often result from the mucosal damage that's secondary to the pre-transplantation chemotherapy and radiation therapy regimens, together with the immunosuppression of the pre-engraftment period and the infection that's secondary to neutropenia. (4). The complications that occur later during the post-transplantation phase include chronic graft-versus-host disease (GVHD) and proliferative diseases such as post-transplantation lymphoproliferative disease (PTLD) or secondary malignant neoplasm (1, 4).
Despite the fact that gastrointestinal complications remain a major cause of morbidity and mortality in pediatric HSCT recipients, there is only limited data regarding the imaging features of the gastrointestinal complications experienced by these patients (3). Careful evaluation of the imaging findings of these gastrointestinal complications, together with the patient's clinical history, allows radiologists to arrive at a more probable differential diagnosis.
Neutropenic colitis is also termed typhlitis (greek: typhlon = cecum), and this is a complication of severe neutropenia in patients who have undergone chemotherapy. Bacterial infection of the chemotherapy-related, damaged mucosa, and often this is superinfection, e.g., Clostridia, may lead to necrotizing inflammatory disease of the ileocecal region (1). The clinical manifestations are a triad of abdominal pain or tenderness, fever and neutropenia, and this all usually improves after neutrophil recovery.
The imaging feature of typhlitis on plain radiography is a paucity of gas in the right side of the abdomen. Dilatation of a small bowel loop suggests mechanical ileus caused by marked ileocecal wall thickening and hypervascularity of the thickened wall that's caused by a transmural inflammatory reaction (4) (Figs. 1A, B). The CT images may demonstrate marked hypodense ileocecal wall thickening, increased mucosal enhancement and inflammatory stranding in the pericecal fat (Fig. 1C). These abnormalities are localized in the cecum and ascending colon, appendix and terminal ileum. Free abdominal gas and fluid collections may occur in those patients with perforations and severe typhlitis (1, 4).
Acute or chronic inflammatory disease of the ileocecal area and the right colon, including cytomegalovirus infection, bacterial ileocecitis, pseudomenbranous enterocolitis and appendicitis, should be considered when making the differential diagnosis. The appropriate microbiological tests for bacteria and viruses include cytomegalovirus (CMV) polymerase chain reaction and the CMV early antigen tests. The endoscopic approach during pancytopenia is usually contraindicated. The finding of a thickened bowel wall involving the ileocecal region, which is associated with the clinical triad of neutropenic patients who have undergone intensive chemotherapy, suggests this diagnosis.
Bacterial pathogens are the predominant cause of infection during the first month following HSCT. The use of antimicrobial agents during this period provides effective preventative treatment, but these drugs can alter the microflora of the colon and cause overgrowth of Clostridium difficile. PMC almost always occurs in children with neutropenia and antibiotic-related diarrhea (4).
The US features of PMC include exaggerated and inhomogenously thickened submucosa with apposition of the mucosal surfaces of the thickened wall (Fig. 2A). The characteristic CT feature of PMC is diffuse colonic wall thickening with low attenuation and trapped contrast media between the thickened haustral folds, and this is referred to as the "accordion sign" (Figs. 2B, C). Pancolonic involvement is common in patients with PMC, but this involvement may be focal or restricted to the right or rectosigmoid colon.
Pseudomembranous colitis can be confirmed by either endoscopic demonstration of the characteristic yellow plaque (pseudomembrane) on the rectal or colonic mucosa or by serologic documentation of Clostridium difficile in the feces (5).
Viruses such as rotavirus, adenovirus and CMV are well recognized causes of the diarrhea in HSCT recipients during the early post-transplantation period, and CMV is the leading cause of intra-abdominal infectious complications. CMV gastroenteritis and hepatitis are major causes of infection-associated mortality following transplantation (4). The imaging findings include non-specific bowel wall thickening, ascites and adjacent inflammatory change, especially in the ileocecal region (Fig. 3).
Graft-versus-host disease results from the damage that donor lymphocytes do to the recipient's target organs. The most commonly affected organs are the skin, liver and gastrointestinal tract. GVHD develops in 55% of the allogenic marrow recipients. The degree of histocompatibility between the stem cell donor and the recipients affects the risk for developing GVHD.
Graft-versus-host disease is not only a primary complication, but it also acts as a predisposing factor for other complications. Because the GVHD-involved gut mucosa impairs the mucosal lymphoid intestinal immunity, patients with GVHD are also susceptible to concomitant gastrointestinal infections.
Acute GVHD usually develops 3-5 weeks after transplantation and it is most commonly diagnosed in conjunction with or it is usually heralded by such skin manifestations as maculopapular rash and pruritis. Chronic GVHD can develop following acute GVHD and it can arise several months after allogenic transplantation (6, 7). It is rare to diagnose GVHD when there is only a single site of involvement such as isolated intestinal tract involvement. Symptoms of intestinal GVHD can also occur, including secretory diarrhea, fever, nausea, vomiting, abdominal pain and intestinal hemorrhage (8).
The plain radiographic findings of intestinal GVHD include air fluid levels, bowel wall thickening, a gasless abdomen, bowel dilatation and ascites. US shows multiple, fluid-filled, dilated bowel loops (Fig. 4). The CT findings that suggest acute GVHD include abnormal bowel wall enhancement, and this correlates histopathologically with mucosal destruction and its replacement by a thin layer of granulation tissue (Figs. 5A, B) (7, 8). As oral contrast material, the use of a negative contrast agent, such as water, may be advantageous for identifying mucosal enhancement. Bowel wall thickening may involve any part of the small or large bowel, but this is less severe than that found in patients with typhlitis or pseudomembranous colitis (4). The common extraintestinal findings of intestinal GVHD include ascites and engorgement of the mesenteric vessels; these findings are more pronounced adjacent to the thickened bowel loops (Figs. 5A, B). The abnormal findings that represent periportal edema and abnormal enhancement of the gall bladder and urinary bladder wall may also occur (Fig. 5C).
Because differentiation of intestinal GVHD from infective enteritis is critical for proper patient treatment, tissue sampling via biopsy is often required for confirmation. Microbiological tests for bacteria and viruses, such as those performed on rectal biopsies, are useful for making the diagnosis of intestinal GVHD. A diagnostic biopsy from skin, liver or rectum is required.
To diagnose the symptoms of intestinal GVHD, as well as the other typical clinical findings of GVHD.
The factors contributing to the development of pneumatosis intestinalis include pre-transplantation chemotherapy and radiotherapy, steroid therapy, infectious colitis, GVHD and septic shock (9). Steroid therapy appears to be a significant factor for the development of pneumatosis in patients who suffer with HSCT. It has been postulated that steroid administration induces atropy of the lymphoid aggregates (Peyer patches) in the gastrointestinal (GI) tract and that the resultant mucosal defects allow dissection of intraluminal air into the submucosal or subserosal regions (2). If this is detected in an asymptomatic patient after steroid therapy, it is usually not associated with bowel necrosis and it can be resolved with conservative management. In these benign cases, even the subsequent development of retroperitoneal air or pneumoperitoneum does not necessitate surgery or indicate a grave prognosis (Fig. 6). However, pneumatosis intestinalis associated with neutropenic colitis is very worrisome as it implies imminent bowel perforation.
The imaging features of pneumatosis intestinalis include bubbly and linear intraluminal lucencies that represent submucosal and subserosal gas, respectively (Fig. 6). Usually, the right side of the colon is predominantly involved.
Thrombotic microangiopathy represents intimal injury of the microvasculature followed by pathophysiologic formation of microthrombi. As intestinal TMA after HSCT is a rare, but fatal complication that occurs in patients who undergo allogenic or autologous BMT (bench mark test), its early diagnosis is very important. The risk factors such as acute GVHD, cyclosporine A (CsA), tacrolimus (FK 506) and total body irradiation may contribute to its occurrence, but the pre-transplant conditioning regimen and the presence of infection may also have a role in the pathogenesis of TMA (10). The intestinal tract is a prevalent site for post-HSCT TMA, and ischemic enterocolitis caused by microangiopathy is another mimic of gut GVHD. Although the clinical symptoms and imaging features of intestinal TMA are similar to those of intestinal GVHD, differentiating TMA from acute GVHD is important for patients suffering from severe and refractory diarrhea after HSCT (10). Because of the difficulty in making a definitive diagnosis of TMA in HSCT recipients, this condition is usually diagnosed based on the clinical and laboratory findings such as the serum lactate dehydrogenase (LDH) levels and the percentage of fragmented erythrocytes. However, as these findings are frequently non-specific, pathologic examination of the gastrointestinal tract may be essential in order to make a definite diagnosis of TMA in HSCT recipients with refractory diarrhea (11). In our series, a 13-year-old girl developed intestinal TMA followed by GVHD. Despite that her skin-GVHD improved with intensive treatment, her intestinal symptom of severe bloody refractory diarrhea progressed. This patient also experienced subsequent hyperammonemic encephalopathy and an elevated level of LDH with fragmented erythrocytes. On the basis of the clinical and laboratory data, this patient was diagnosed with CsA-associated neurotoxicity with microangiopathic hemolytic anemia. To the best of our knowledge, little attention has been given to the imaging features of intestinal TMA after transplantation and although they do not differ from those of GVHD, intraluminal or intramucosal hemorrhage is frequently present in the TMA patients who present with severe bloody diarrhea (Fig. 7).
Post-transplantation lymphoproliferative disease is caused by Epstein-Barr virus, which induces uncontrolled proliferation of engrafted B lymphocytes in organ transplant recipients. The histologic subtypes of PTLD range from polyclonal lymphoid hyperplasia to malignant monoclonal lymphoma (12). GI tract involvement by PTLD frequently occurs, but this is rare following HSCT, with a prevalence of 0.34% and 1.4% in patients with matched allogenic grafts and unmatched grafts, respectively (13).
The imaging features of PTLD are similar those of non-Hodgkin's lymphoma. PTLD involving the GI tract may be infiltrative, and so it causes circumferential mural infiltration with luminal excavation or there is a discrete eccentric mass that causes luminal narrowing with intussusception (12) (Fig. 9).
Reduced immunosuppression in the case of polyclonal hyperplasia leads to regression of the PTLD, whereas aggressive chemotherapy is required for monoclonal lymphoma. Unlike the PTLD in solid organ recipients, the PTLD seen in HSCT recipients has a poor prognosis because decreasing these patients' immunosuppression is not a feasible therapeutic option.
Hematopoietic stem cell transplantation has resulted in the prolonged survival of children with hematologic malignancy. However, gastrointestinal complications following transplantation are now seen as a major cause of morbidity and mortality in pediatric HSCT recipients. Therefore, it is important to recognize the imaging findings of these complications in those patients who undergo hematopoietic stem cell transplantation in order to identify and properly treat them.
Figures and Tables
Fig. 1
Neutropenic colitis in 10-year-old boy, and this developed on 23rd day after bench mark test for treating his leukemia.
A, B. US of right lower guadrant abdomen shows marked asymmetric echogenic wall thickening and abundant vascular flow of cecum (A) and terminal ileum (B).
C. CT scan shows thickening of cecal wall with associated luminal narrowing and stranding in pericecal fat (arrow).
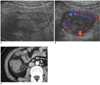
Fig. 2
Pseudomembranous colitis in 9-year-old boy, and this developed on 23rd day after bench mark test for treating his leukemia.
A. Longitudinal US of ascending colon shows rather striking diffuse thickening of colonic wall (arrows). Exaggerated haustral markings and inhomogenously thickened submucosa with apposition of muscosal surfaces of thickened wall are noted.
B, C. Axial contrast enhanced CT shows pancolitis involving ascending, transverse, descending (B) and rectosigmoid colon (C). Note the hyperemic enhancing mucosa surrounded by thickened hypodense submucosa edema, which forms accordion pattern (arrows).
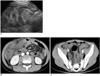
Fig. 3
Cytomegalovirus enteritis in 15-year-old boy, and this developed during second month after bench mark test for treating his aplastic anemia.
A, B. US of abdomen shows multiple sites of segmental hypoechoic bowel wall thickening that involves terminal ileum (A), and there are abundant Doppler signals with ascites (B).
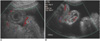
Fig. 4
Acute graft-versus-host disease in 12-year-old boy, and this developed on 32nd day after bench mark test for treating his leukemia.
A, B. US of abdomen shows slightly thickened echogenic wall (arrows) (A) and fluid (*) distended small bowel loops with ascites (B).
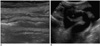
Fig. 5
Acute graft-versus-host disease in 11-year-old-boy, and this developed on 40th day after bench mark test for treating his leukemia.
A, B. Axial contrast-enhanced CT scans show ascites and diffuse wall thickening with mucosal enhancement involving small bowel (long arrows) and large bowel (short arrows).
C. Coronal MPR contrast-enhanced CT demonstrates more pronounced engorgement of vasa recta (arrow) adjacent to thickened bowel wall segment, as well as multiple, fluid-filled, dilated loops of colon without wall thickening in sigmoid colon (*). Abnormal enhancement of gall bladder and urinary bladder is also present, and this is similar to that seen within bowel wall.
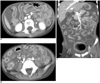
Fig. 6
Benign pneumatosis intestinalis in 15-year-old asymptomatic boy, and this developed on 75th day after BMT for treating his leukemia.
A. Plain film of abdomen shows diffuse linear and bubbly intramural air along entire colon, as well as presence of subhepatic gas.
B. CT scan shows diffuse intramural air densities in entire colon with involvement of retroperitoneal (arrows) and intraperitoneal cavities.
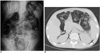
Fig. 7
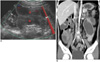
Intestinal thrombotic microangiopathy followed by graft-versus-host disease in 13-year-old girl, and this developed on 28th day after peripheral blood stem cell transplantation for treating her leukemia.
A, B. US shows multiple distended small bowel loops with wall thickening. Medium echogenic debris with floating echogenicities that fill distended lumen are noted in some of these loops (*).
C. Unenhanced coronal multiplanar reconstruction CT image shows increased intraluminal attenuation, which is consistent with intraluminal bleeding (*), and submucosal zone of decreased attenuation parallel to lumen (arrowhead). Ascites and mesenteric thickening are also noted.
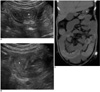
Fig. 8
Megacolon in 14-year-old boy with neutropenic colitis, and this developed on 39th day after peripheral blood stem cell transplantation for treating his leukemia.
A. Initial CT shows diffuse concentric thickening with increased vascularity involving cecum and ascending colon (arrows). Lumen is distended and partially fluid-filled.
B. Follow-up CT one month later shows resolution of previous wall thickening, but there is also marked fluid/gas-distended right side of colon, which appears as megacolon (arrows).
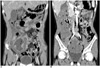
Fig. 9
Post-transplantation lymphoproliferative disease involving small bowel in five-year-old boy, and this developed during third year following bench mark test for treating his leukemia.
A. Longitudinal US of left mild abdomen shows short area of segmental severe concentric hypoechoic mural thickening (*), and this causes luminal narrowing in jejunum.
B. CT scan shows short area of segmental concentric wall thickening with homogenous enhancement of small bowel loop in left side of abdomen (arrow).
References
1. Benya EC, Sivit CJ, Quinones RR. Abdominal complications after bone marrow transplantation in children: sonographic and CT findings. AJR Am J Roentgenol. 1993. 161:1023–1027.
2. Jones B, Fishman EK, Kramer SS, Siegelman SS, Saral R, Beschorner WE, et al. Computed tomography of gastrointestinal inflammation after bone marrow transplantation. AJR Am J Roentgenol. 1986. 146:691–695.
3. Barker CC, Anderson RA, Sauve RS, Butzner JD. GI complications in pediatric patients post-BMT. Bone Marrow Transplant. 2005. 36:51–58.
4. Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005. 25:305–317.
5. Bolton RP, Thomas DF. Pseudomembranous colitis in children and adults. Br J Hosp Med. 1086. 35:37–42.
6. Donnelly LF, Morris CL. Acute graft-versus-host disease in children: abdominal CT findings. Radiology. 1996. 199:265–268.
7. Mentzel HJ, Kentouche K, Kosmehl H, Gruhn B, Vogt S, Sauerbrey A, et al. US and MRI of gastrointestinal graft-versus-host disease. Pediatr Radiol. 2002. 32:195–198.
8. Fisk JD, Shulman HM, Greening RR, McDonald GB, Sale GE, Thomas ED. Gastrointestinal radiographic features of human graft-vs.-host disease. AJR Am J Roentgenol. 1981. 136:329–336.
9. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988. 151:85–87.
10. Nishida T, Hamaguchi M, Hirabayashi N, Haneda M, Terakura S, Atsuta Y, et al. Intestinal thrombotic microangiopathy after allogeneic bone marrow trasplanatation: a clinical imitator of acute enteric graft-versus-host disease. Bone Marrow Transplant. 2004. 33:1143–1150.
11. Narimatsu H, Kami M, Hara S, Matsumura T, Miyakoshi S, Kusumi E, et al. Intestinal thrombotic microangiopathy following reduced-intensity umbilical cord blood transplantation. Bone Marrow Transplant. 2005. 36:517–523.
12. Lim GY, Newman B, Kurland G, Webber SA. Post transplantation lymphoproliferative disorder: manifestations in pediatric thoracic organ recipients. Radiology. 2002. 222:699–708.
13. Lones MA, Kirov I, Said JW, Shintaku IP, Neudorf S. Post-transplant lymphoproliferative disorder after autologous peripheral stem cell transplantation in a pediatric patient. Bone Marrow Transplant. 2000. 26:1021–1024.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download