Abstract
Positron emission tomography combined with computed tomography (PET/CT) has been receiving increasing attention during the recent years for making the diagnosis, for determining the staging and for the follow-up of various malignancies. The PET/CT findings of 58 breast cancer patients (age range: 34-79 years old, mean age: 50 years) were retrospectively compared with the PET or CT scans alone. PET/CT was found to be better than PET or CT alone for detecting small tumors or multiple metastases, for accurately localizing lymph node metastasis and for monitoring the response to chemotherapy in breast cancer patients.
Imaging modalities like computed tomography (CT) and magnetic resonance (MR) imaging rely on detecting anatomic changes for the diagnosis, staging and follow-up of cancer patients. However, positron emission tomography (PET) has the ability to demonstrate abnormal metabolic activity, and 18F-2-deoxy-D-glucose (FDG) PET provides important tumor-related qualitative and quantitative metabolic information that may be critical for the diagnosis and follow-up (1-3). Moreover, the combination of PET and computed tomography (PET/CT) allows the functional PET and anatomical CT images to be acquired under identical conditions and then they are rapidly co-registered. This combined system has advantages over CT alone as functional information is added to morphological data, and this combined system has advantages over PET alone because pathological areas of tracer uptake are better localized and the image acquisition time is reduced (4-7). Moreover, the limited specificity of PET that's due to the increased glucose metabolic activities of benign tumors and inflammatory tissues (such as those of tuberculosis) can be partially overcome by PET/CT (8, 9).
In this study, we investigated the role of PET/CT for evaluating breast cancer.
Fifty-eight female patients with known breast cancer (age range: 34-79 years old, mean age: 50 years), and who underwent PET/CT examinations at our facility between April 2003 and June 2005 were enrolled in this study. The PET/CT examinations were performed for the initial tumor staging, for suspected recurrence after breast operation and for follow-up after chemotherapy.
Whole-body PET/CT imaging was performed using a combined PET/CT scanner (Gemini, Philips Medical Systems, Bothell, WA). The Gemini is an open PET/CT system that combines a helical dual slice CT scanner and a 3D PET scanner equipped with its own transmission source. The MX8000 EXP CT scanner is a dual slice system with a detector that consists of 1,344 cadmium tungstate elements. After having the patients fast for at least four hours, they were administered an intravenous injection of 240 to 400 MBq (approximately 10 mCi) FDG; oral contrast was not administered. The blood glucose levels were checked in all the patients before FDG administration, and no patient had a blood glucose level that exceeded 200 mg/dl. The CT images were acquired from the cerebellum to the pelvis without intravenous contrast media, and a whole-body emission PET scan was performed for the same axial coverage. The PET, CT and fused PET/CT images were generated and then reviewed on a computer workstation.
The ability of PET to detect breast cancer depends on the tumor's size and histology. The sensitivity of PET has been reported to be 68% for small (< 2 cm) tumors and 92% for larger (2-5 cm) tumors (10), and its reported overall accuracy for detecting in situ carcinomas is low (sensitivity: 2-25%). The major limitation of PET or PET/CT for breast imaging is its poor detection rate for small breast carcinomas and non-invasive breast cancers (Fig. 1).
However, PET/CT has a role to play in a selected group of patients, such as those with dense breasts or with implants, for determining tumor multiplicity, for localizing the primary tumor in those patients with metastases of a breast origin when the mammography is indeterminate, and for those patients whom biopsy is not a desirable option (11, 12). PET/CT has a potential advantage over PET for evaluating small lesions in which the uptake may be artifactually lowered due to the partial volume effect of PET because areas of mild hyperglycolytic activity can be reliably assigned to normal or abnormal anatomical structures (Fig. 2).
Axillary lymph node metastasis is an important factor when determining the prognosis of patients. Breast cancer patients with four or more involved axillary lymph nodes have a significantly higher risk of recurrence (13). The sensitivity and specificity of axillary PET imaging in breast cancer patient have been reported as 79-94% and 86-92%, respectively (11, 14). PET/CT can accurately localize and differentiate the metastatic and reactive lymph nodes when CT shows multiple enlarged lymph nodes in the axilla (Fig. 3).
The metastasis to the internal mammary or mediastinal lymph nodes in breast cancer patients is often clinically occult. The prevalence of abnormal findings for the internal mammary or mediastinal lymph nodes by PET was about twice than of conventional CT in those patients with recurrent or metastatic breast cancer (15). In a study by Tatsumi et al., although the sample number was small, PET/CT appeared to be more useful than CT for evaluating the internal mammary and mediastinal lymph nodes because the smaller lymph nodes sometimes produced equivocal or negative CT findings (7) (Fig. 4).
Distant metastases from breast cancer are frequently found in the lungs, liver and bones. One advantage of whole-body PET imaging over conventional imaging modalities such as chest films, bone scanning, and abdominal ultrasound is its ability to detect metastasis at different sites and organs during a single examination (Fig. 5). Moon et al. found that whole-body PET imaging had high diagnostic accuracy for patients with suspected recurrent or metastatic breast carcinoma (16). Based on the number of lesions, its sensitivity for detecting distant metastasis was 85% and its specificity was 79%.
Cook et al. found that PET was superior to bone scintigraphy for detecting osteolytic breast cancer metastases (17) (Fig. 6). In contrast, the osteoblastic metastases showed lower metabolic activity and they were frequently undetectable by PET (17, 18). Yet PET/CT can overcome this limitation, and osteoblastic bone lesions, even if negative on PET scans, can be identified on CT images (Fig. 7).
To downstage primary tumors prior to surgery and to abolish occult distant metastases, neoadjuvant chemotherapy is now being increasingly used to manage patients with large and locally advanced breast cancer. Moreover, several studies have demonstrated that patients with unresponsive tumor may achieve improved survival by administering alternative chemotherapy and/or prolonged courses of chemotherapy (19, 20). Considering the side effects of chemotherapy, there is a need to quickly identify the non-responding patients early in their treatment.
At present, the anatomical imaging modalities are commonly used to evaluate the response to treatment by evaluating the changes of the tumor's size. Nevertheless, sequential measurement of tumor size frequently does not allow the determination of early response. The effect of PET for evaluating the response to treatment has already been demonstrated for different types of neoplasm, including breast cancer (Fig. 8). In a study by Smith et al, the mean reduction in FDG uptake after the first cycle of chemotherapy was significantly higher in the lesions that showed a partial (p = 0.013), complete macroscopic (p = 0.003), or complete microscopic (p = 0.001) response than that of the non-responsive lesions, as determined by histopathological examinations (20). Rose et al. have reported that after a single cycle of chemotherapy, PET predicted the complete pathological response with a sensitivity of 90% and specificity of 74%, and by using a decrease of FDG uptake at the threshold of < 55%, as compared with the baseline scan, all the responders were correctly identified after the first treatment course (100% sensitivity and 85% specificity) (21).
PET/CT may also play a role in radiation therapy planning by providing an accurate estimate of the extent of tumor (22).
Detecting early recurrence has an important survival benefit because it prompts clinical consideration for administering different therapies. However, it is difficult to differentiate true recurrence from postsurgical sequelae and radiation sequelae with using just the conventional imaging modalities. Locoregional recurrences predominately affect the breast, skin, the axillary and supraclavicular nodes, and the chest wall (23, 24).
Grahek et al. studied 134 patients with suspected recurrence and they found that the sensitivity and specificity of PET for detecting recurrence were 84% and 78%, respectively, whereas the sensitivities and specificities of the conventional imaging modalities were 63% and 61%, respectively (25). PET is considered to be highly effective for evaluating patients with suspected recurrent breast cancer, and it surpasses the other conventional imaging modalities in terms of whole-body evaluation. The CT data from a PET/CT examination allows the appropriate anatomical localization of foci of FDG uptake (Fig. 9).
Figures and Tables
Fig. 1
Ductal carcinoma in situ in a 49-year-old woman.
A. Sonography shows a 2.5 cm sized hypoechoic mass with an indistinct margin in the left upper breast (arrows).
B. The PET/CT image shows no evidence of FDG uptake in the left breast. Surgery revealed ductal carcinoma in situ.
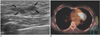
Fig. 2
Invasive breast cancer in a 57-year-old woman.
A. The mediolateral oblique view of the screening mammogram shows a 1.1 cm sized spiculated mass (arrow) in the left breast.
B. The PET image shows faint FDG uptake (SUV = 1.2) (arrow) in the left breast. It is difficult to detect the lesion due to partial volume averaging.
C. The PET/CT image shows that the focal uptake (arrow) is localized to the left breast.
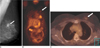
Fig. 3
Axillary lymph node metastasis in a 45-year-old woman with a 4 cm invasive ductal carcinoma.
A. The PET image shows increased FDG uptake in the right breast (black arrow) and axilla (white arrow).
B. The CT image shows two enlarged lymph nodes in the right axilla (arrows).
C. The PET/CT image shows accurate localization of the metastatic (white arrow, SUV = 9.9) and reactive (black arrow) lymph nodes. Surgery revealed one metastatic lymph node out of the 21 excised axillary lymph nodes.
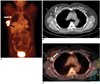
Fig. 4
Mediastinal lymph node metastasis in a 41-year-old woman who had undergone left modified radical mastectomy 10 months previously.
A. The PET image shows multiple areas of increased uptake (arrows) in the left upper chest.
B. The CT image shows a small soft tissue density in the anterior mediastinum (arrow).
C. The PET/CT image shows co-registration of the FDG uptake and the soft tissue density in the anterior mediastinum, suggesting internal mammary lymph node metastases (arrow).
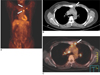
Fig. 5
Multiple distant metastases in a 44-year-old woman with bilateral breast cancer.
A. The PET image shows multiple areas of FDG uptake in the thorax and abdomen.
B, C. The PET/CT images show high uptake in both breasts (white arrows in B), the mediastinal lymph nodes (black arrows in B) and the visceral organs (arrows in C).
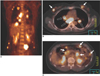
Fig. 6
Bone metastases in a 47-year-old woman with breast cancer.
A-C. The PET and PET/CT images show increased FDG uptake in the T5 (B) and L1 (C) vertebrae (arrows). Osteolytic changes suggestive of bone metastases are seen on CT.
D. The whole body Tc-99m bone scintigram obtained 7 days before the PET scan shows faint uptake in only the L1 vertebra (arrow). T5 metastasis is not visualized.
E. The follow-up bone scintigram obtained three months later shows foci of hot uptake in the T5 and L1 vertebrae (arrows). PET/CT was superior to bone scintigram for the early detection of osteolytic breast cancer metastasis.
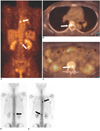
Fig. 7
Bone metastases in a 64-year-old woman who had undergone right modified radical mastectomy 36 months previously.
A. The bone scintigram shows increased FDG uptake in the right 1st and left 7th ribs (arrow), which is probably due to bony metastases.
B. The PET/CT image shows no FDG uptake in the left 7th rib (arrow).
C. The CT image shows an osteoblastic bony lesion in the left 7th rib (arrow).
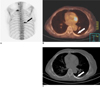
Fig. 8
Chemotherapy in a 35-year-old woman with bilateral breast cancer and bone metastases.
A-C. The initial PET (A, B) and PET/CT (C) images show strong FDG uptake (arrows) in both breasts and in multiple spinal levels.
D-F. The follow-up PET (D, E) and PET/CT (F) images obtained after three cycles of chemotherapy show markedly decreased FDG uptake in both breasts and in multiple spinal levels.
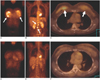
Fig. 9
Local recurrence in a 74-year-old woman who had undergone right modified radical mastectomy eight years previously.
A. Sonography shows an 1.4 cm oval mass with increased vascularity in the right pectoralis muscle at the mastectomy site.
B, C. The PET images show focal high FDG uptake (SUV= 3.3) (arrows) in the right chest wall.
D. The PET/CT image shows a focus of high FDG uptake (arrow) localized to the right pectoralis muscle. Accurate localization of the lesion was difficult with using PET alone.
References
1. Minn H, Soini I. F-18 fluorodeoxyglucose scintigraphy in diagnosis and follow up of treatment in advanced breast cancer. Am J Clin Pathol. 1989. 91:535–541.
2. Kubota K, Matsuzawa T, Amemiya A, Kondo M, Fujiwara T, Watanuki S, et al. Imaging of breast cancer with F-18 fluorodeoxyglucose and positron emission tomography. J Comput Assist Tomogr. 1989. 13:1097–1098.
3. Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991. 179:765–770.
4. Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med. 2003. 44:224–239.
5. Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000. 41:1369–1379.
6. Ell PJ, Von Schulthess GK. PET/CT: a new road map. Eur J Nucl Med Mol Imaging. 2002. 29:719–720.
7. Tatsumi M, Cohade C, Mourtzikos KA, Fishman EK, Wahl RL. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging. 2006. 33:254–262.
8. Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging. 2004. 31:Suppl1. S125–S134.
9. Fueger BJ, Weber WA, Quon A, Crawford TL, Allen-Auerbach MS, Halpern BS, et al. Performance of 2-Deoxy-2-[F-18]fluoro-D-glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol. 2005. 7:369–376.
10. Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000. 18:3495–3502.
11. Schirrmeister H, Kühn T, Guhlmann A, Santjohanser C, Hörster T, Nüssle K, et al. Fluorine-18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med. 2001. 28:351–358.
12. Noh DY, Yun IJ, Kang HS, Kim YC, Kim JS, Chung JK, et al. Detection of cancer in augmented breast by positron emission tomography. Eur J Surg. 1999. 165:847–851.
13. Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003. 44:1200–1209.
14. Greco M, Crippa F, Agresti R, Seregni E, Gerali A, Giovanazzi R, et al. Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-D-glucose positron emission tomography: clinical evaluation and alternative management. J Natl Cancer Inst. 2001. 93:630–635.
15. Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. 18fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001. 19:3516–3523.
16. Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998. 39:431–435.
17. Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998. 16:3375–3379.
18. Uematsu T, Yuen S, Yukisawa S, Aramaki T, Morimoto N, Endo M, et al. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005. 184:1266–1273.
19. Heys SD, Eremin JM, Sarkar TK, Hutcheon AW, Ah-See A, Eremin O. Role of multimodality therapy in the management of locally advanced carcinoma of the breast. J Am Coll Surg. 1994. 179:493–504.
20. Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000. 18:1676–1688.
21. Rése C, Dose J, Avril N. Positron emission tomography for the diagnosis of breast cancer. Nucl Med Commun. 2002. 23:613–618.
22. Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004. 59:78–86.
23. Eubank WB, Mankoff DA, Vesselle HJ, Eary JF, Schubert EK, Dunnwald LK, et al. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. RadioGraphics. 2002. 22:5–17.
24. Kamby C, Vejborg I, Kristensen B, Olsen LO, Mouridsen HT. Metastatic pattern in recurrent breast cancer. special reference to intrathoracic recurrences. Cancer. 1988. 62:2226–2233.
25. Grahek D, Montravers F, Kerrou K, Aide N, Lotz JP, Talbot JN. [18 F]FDG in recurrent breast cancer: diagnostic performances, clinical impact and relevance of induced changes in management. Eur J Nucl Med Mol Imaging. 2004. 31:179–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download