Abstract
Objective
To evaluate the MR imaging findings of painful type II accessory navicular bone and to correlate these with the surgical and pathologic findings.
Materials and Methods
The MR images of 17 patients with medial foot pain and surgically proven type II accessory navicular abnormalities were reviewed. The changes of signal intensity in the accessory navicular, synchondrosis and adjacent soft tissue, the presence of synchondrosis widening, and posterior tibial tendon (PTT) pathology on the T1-weighted and fat-suppressed T2-weighted images were analyzed. The MR imaging findings were compared with the surgical and pathologic findings.
Results
The fat-suppressed T2-weighted images showed high signal intensity in the accessory navicular bones and synchondroses in all patients, and in the soft tissue in 11 (64.7%) of the 17 patients, as well as synchondrosis widening in 3 (17.6%) of the 17 patients. The MR images showed tendon pathology in 12 (75%) of the 16 patients with PTT dysfunction at surgery. The pathologic findings of 16 surgical specimens included areas of osteonecrosis with granulomatous inflammation, fibrosis and destruction of the cartilage cap.
Conclusion
The MR imaging findings of painful type II accessory navicular bone are a persistent edema pattern in the accessory navicular bone and within the synchondrosis, indicating osteonecrosis, inflammation and destruction of the cartilage cap. Posterior tibial tendon dysfunction was clinically evident in most patients.
The accessory navicular bone is one of several accessory ossicles of the foot and is considered as a normal anatomic and radiographic variant (1-10). Accessory navicular bones are classified into three types based on their shape and location in relation to the navicular bone (3-6). Type I is a 2-3 mm sized sesamoid bone in the posterior tibial tendon (PTT) and is referred to as "os tibiale externum" and accounts for approximately 30% of all accessory navicular bones. Type II is a secondary ossification center of the navicular bone and is also referred to as "prehallux", accounting for approximately 50-60% of accessory navicular bones. It is seen over the medial pole of the navicular bone at between nine and 11 years of age (3). On radiographs, this ossicle is triangular or heart-shaped, approximately 9×12 mm in size, with its base situated 1-2 mm from the medial and posterior aspects of the navicular bone. It is connected to the navicular tuberosity by a fibrocartilage or a hyaline cartilage layer. Type III is a prominent navicular tuberosity and is considered as a fused variant of the type II accessory navicular bone (3-7). Type II accessory navicular bone may be symptomatic and cause medial foot pain (7, 8). Even though type II accessory navicular bones may be symptomatic, plain radiographs may be not helpful in their diagnosis. On the other hand, bone scintigraphy or MR imaging is helpful (5-9).
The MR imaging findings of painful accessory navicular bones are known to comprise altered signal intensity and a bone marrow edema pattern, which are suggestive of chronic stress and/or osteonecrosis (5, 6, 8). Additional radiological findings, such as PTT tear and pathologic findings of excised accessory navicular bones, have also been reported, but there have been few reports in which the MR imaging results were correlated to the pathologic findings (5, 6, 9-18).
The purpose of this study is to determine the MR imaging findings of painful type II accessory navicular bones and to evaluate the relationship between these and the surgical and pathologic findings.
The MR images of 17 patients (10 male, 7 female; mean, 27 years; range, 13-56 years) with chronic medial foot pain and surgically proven type II accessory navicular abnormalities, which were treated in our institution between January 1998 and December 2002, were reviewed. In all cases, the patients' histories were obtained and the physical examinations were performed by an experienced orthopedic foot surgeon. Five of the 17 patients were middle-aged women (age range was 43-56 years with a mean of 49.6 years). These 17 patients had experienced pain for a mean duration of 18 months (range, 5 months-5 years). In five cases, the patients reported bilateral medial foot pain, but MR imaging was only performed unilaterally. Eight (47%) of the 17 patients had a history of sporting activity and five (29.4%) had a history of trauma. None of the patients had other associated medical problems.
Surgical treatment consisted of excision of the type II accessory navicular bones, synchondrosis and the adjacent margin of the navicular, in conjunction with repositioning of the PTT in 12 of the 17 patients, excision of the accessory bones with flexor digitorum longus (FDL) transfer in two, excision of the accessory bones with FDL transfer and slide calcaneal osteotomy in two, and triple fusion in one patient, who had a complete PTT tear.
MR imaging was performed using a 1.5-T system (Signa; GE Medical Systems, Milwaukee, Wis) and a phased array extremity coil in the axial, coronal and sagittal planes parallel to the top of the table. The patients were situated in the supine position with their feet positioned so as to have approximately 15-20 degrees of plantar flexion. Axial T1-weighted spin-echo (SE) (500-600/11-15 [TR msec/TE msec]) and frequency-selective fat-suppressed T2-weighted fast SE (FSE) (4000/95-132 [TR/effective TE]) images with an echo train length of eight, sagittal T1-weighted SE (500-600/11-15) and T2-weighted FSE (4000/95-100) images, and coronal T1-weighted SE images (500-600/11-15) were acquired. Axial T2-weighted FSE images (4000/95-144) were acquired in 7 patients. The field of view was 12-16 cm, the section thickness was 3-4 mm with 0-1 mm intervals and the matrix size was 256×192.
All of the MR images were retrospectively reviewed in a blind fashion, with agreement being obtained by consensus in all cases between two musculoskeletal radiologists. We evaluated the changes of signal intensity in the accessory navicular bones, synchondrosis and adjacent soft tissue on the T1-weighted images and fat-suppressed T2-weighted image (WI). The changes in the signal intensity were compared to those of the adjacent fatty marrow. We also evaluated the presence of synchondrosis widening (a base more than 2 mm from the medial and posterior aspect of the navicular bone) and PTT pathology, such as tenosynovitis (significant fluid in the tendon sheath, normal sized and normal signal intensity tendon), tendinosis (tendon thickening with increased intra-substance signal intensity on the T1WI and fat-suppressed T2WI) and tear. On the MR images, the PTT tears were divided into partial (types I and II) and complete (type III), according to the classification of Rosenberg. The type I tears represented partial ruptures with a longitudinal split and tendon enlargement, while the type II tears represented partial rupture with a decrease in the size of the tendon, and the type III tears signified complete disruption.
The MR imaging findings were compared with the surgical and pathologic findings. The time interval between the MR examination and surgery ranged from seven days to five months (mean, 50 days).
The fat-suppressed T2-weighted FSE images showed high signal intensity accessory navicular bones and synchondroses resembling bone marrow edema patterns in all patients (Figs. 1-3). The high signal intensity was most intense adjacent to the synchondrosis and was also observed in the nontendinous soft tissue adjacent to the accessory navicular bones on the fat-suppressed T2-weighted FSE images in 11 (64.7%) of the 17 patients. The synchondrosis had widened in three (17.6%) of the 17 patients, and these three patients showed mobile accessory navicular bones at surgery.
The pathologic findings in 16 surgical specimens included areas showing osteonecrosis with granulomatous inflammation and fibrosis and new bone formation. There was also destruction of the cartilage cap in 14 of the 15 surgical specimens (93.3%) which had a cartilage cap available (Fig. 1D), and this was observed as high signal intensity on the fat-suppressed FSE images. In addition, there was inflammation of the synovium in four of these specimens (Table 1).
The MR images showed tendon pathology in 12 (75%) of the 16 patients who were found to have PTT dysfunction at surgery. The MR imaging findings were as follows: tenosynovitis (n=3) (Fig. 2), tendinosis (n=3); partial tendon tear (n=5, type I; n=1, type II; n=4) (Fig. 3); complete tendon tear (type III; n=1). The PTT dysfunctions at surgery were as follows: tenosynovitis (n=4), tendon degeneration (n=6), partial tear with a longitudinal split (n=5) and complete tendon tear (n=1). In addition, a mass of fibrocartilage tissue between the tendon and the accessory navicular bone was present in one patient who had PTT degeneration at surgery.
Pain was relieved by surgery in 15 of the 17 patients, but persisted in one patient, who underwent a triple fusion, and was aggravated in another patient who developed a flatfoot condition after surgery.
The accessory navicular bone presents in 4-21% of the population and is the direct cause of foot pain in some patients (7). The most frequent complaint of type II accessory navicular bone is pain and tenderness. The pain is localized to the medial aspect of the navicular and is aggravated by weight-bearing, walking, athletic activity or the wearing of narrow shoes. The cause of the pain in subjects with type II accessory navicular bone was thought to be repetitive tension and shear stress across the synchondrosis as a result of the pull of the PTT (4). Unless there is early immobilization of the synchondrosis, healing will not occur, and the ensuing chronic injury may lead to cartilage proliferation and bone remodeling at the cartilage-bone interface of the synchondrosis, although synchondroses have shown no abnormal signal intensity on MR imaging in previous reports (3-6, 8, 10, 11). The pathologic analysis of the accessory navicular bones excised in the present study revealed areas of osteonecrosis with granulomatous inflammation and fibrosis, as well as new bone formation. Fourteen of these pathologic specimens also showed frayed cartilage surface, which was observed as altered signal intensity on MR imaging. Necrosis, granulation tissue and new bone formation are suggestive of chronic repetitive injury and repair. Thus, the bone marrow edema pattern observed in painful type II accessory navicular bone and synchondrosis on MR imaging is indicative of osteonecrosis, inflammation and destruction of the cartilage cap, which is compatible with chronic stress-related injury.
The interface of the synchondroses had widened in three patients, and two of these patients had a history of trauma. The trauma might have caused direct injury to the synchondrosis with potentially painful results. These three patients showed mobile accessory navicular bones at surgery. Pathologic analysis revealed severe destruction of the cartilage cap in two of these three patients and it could not be performed in the third patient, because the carilage cap was severely damaged.
The relationship between the PTT and the accessory navicular bone has previously been evaluated (5, 11-19), and it was found that the accessory navicular bone increases the stress on the distal PTT (13). The bulk of the PTT inserts into the accessory ossicle when this is present. This leads to a straightening of the distal tendon and to the PTT acting as an adductor, which interferes with the normal tarsal mechanics, weakens the longitudinal arch and produces a painful flatfoot condition (13, 14). Kiter et al. also reported that the PTT inserted directly into the accessory navicular bone, without extending the sole of the foot. A fibrocartilaginous mass was also detected, whose probable purpose was to ameliorate the effect of friction between the tendon and the bone (17, 18). In our study, one patient showed a fibrocartilaginous mass between the tendon and bone at surgery.
In the present study, PTT dysfunction was present in 16 patients at surgery and MR imaging detected this PTT pathology in 12 of these 16 patients (75%). In one of the four patients with synovitis at surgery, no tendon sheath fluid was observed on T2-weighted imaging. However, it is conceivable for clinically described synovitis not to be manifested on MR imaging. Since synovitis is considered to be an early clinically observable stage in the development of PTT tear, it is also possible that the synovitis in the chronic tears had already resolved or had developed into fibrosis (15, 19). Tendinosis manifests as a change in tendon size or internal signal. In three of the six patients with PTT degeneration at surgery, the PTT had not thickened and manifested itself in the form of an intrinsically normal signal intensity on MR imaging. MR imaging showed tendon tear in all six patients found to have a PTT tear at surgery, but the presumed tear classification made by MR imaging did not correlate with the surgical findings. We encountered some difficulty in reproducing the range of tendon size and appearance in the patients with PTT tears, when this condition was evaluated on MR imaging. In our series, one patient had a complete tear of the PTT and gradual development of a flatfoot condition. Diagnostic overlap may exist between severe PTT tendinosis and type I tear in the distal portion of the tendon on MR imaging, as well as between torn PTT and normal tendon (15, 20, 21). In our series, to decrease the possibility of MR imaging leading to the incorrect diagnosis of the PTT pathology, the MR imaging was done with plantar flexion of the affected foot.
In our series, MR imaging showed an altered signal intensity pattern in all patients and was able to correctly detect the PTT pathology in 75% of the patients with PTT dysfunction. This figure is lower than that found by Rosenberg (22). MR imaging offered the advantage of making it possible to assess the abnormalities of the accessory navicular bones and synchondroses, to identify the PTT pathology and to explain medial foot pain. Since there was not always a good correlation between the MR imaging and surgical findings, the surgical decisions were made on clinical grounds.
We concluded that the MR imaging findings of painful type II accessory navicular bone are a persistent edema pattern in the accessory navicular bone and within the synchondrosis, indicating osteonecrosis, inflammation and destruction of the cartilage cap, with these findings being compatible with chronic stress-related injury. Posterior tibial tendon dysfunction was clinically evident in most patients.
Figures and Tables
Fig. 1
Painful accessory navicular bone in a 14-year-old male soccer enthusiast.
A. Anteroposterior radiograph of right foot shows a type II accessory navicular bone (arrow).
B. Axial T1-weighted spin-echo image (TR/TE, 600/15) shows focal low signal intensity in the medial margin of the accessory navicular bone (arrow).
C. Axial fat-suppressed T2-weighted fast spin-echo image (TR/TE, 4000/132) shows high signal intensity in the accessory navicular bone (long solid arrow), synchondrosis and navicular tuberosity (short solid arrow), which is most intense adjacent to the synchondrosis. At surgery, posterior tibial tendon degeneration was observed.
D. Photomicrograph of excised accessory navicular bone shows destruction of the cartilage cap that represents the synchondrosis (large black arrow), subchondral osteonecrosis (short black arrows) and granulation tissue (white arrows) (H & E stain; original magnification, ×40).
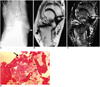
Fig. 2
Surgically proven synovitis with painful accessory navicular bone in a 16-year-old boy.
A. Axial fat-suppressed T2-weighted fast spin-echo image (TR/TE, 4000/108) shows high signal intensity in the accessory navicular bone (long solid arrow) and synchondrosis (short solid arrow).
B. Axial T1-weighted spin-echo image (TR/TE, 600/11) at a level close to the accessory navicular bone shows a posterior tibial tendon (solid arrow) of normal size and signal intensity. Note the decreased signal intensity around the tendon (open arrow)
C. Sagittal T2-weighted fast spin-echo image (TR/TE, 4000/95) shows fluid in the left posterior tibial tendon sheath (open arrows) with accessory navicular bone (solid arrow).
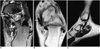
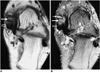
Fig. 3
Surgically proven partial tear of the posterior tibial tendon with painful accessory navicular bone in a 51-year-old woman.
A. Axial T1-weighted spin-echo image (TR/TE, 500/12) shows low signal intensity in the accessory navicular bone (solid arrow) with distraction of the synchondrosis. The posterior tibial tendon is thickened with increased signal intensity in the tendon (open arrow).
B. Fat-suppressed T2-weighted fast spin-echo image (TR/TE, 4000/108) shows high signal intensity in the accessory navicular bone (long solid arrow) and fluid signal intensity in the synchondrosis (short solid arrow). The posterior tibial tendon displays increased signal intensity (open arrow), indicative of partial thickness tear.
Acknowledgments
The authors wish to thank Hollis G. Potter, MD, Chief, Devision of Magnetic Resonance Imaging, Department of Radiology and Imaging, Weill Medical College of Cornell University, for reviewing this manuscript.
References
1. Geist ES. Supernumerary bone of the foot: A roentgen study of the feet of 100 normal individuals. Am J Orthop Surg. 1914. 12:403.
2. Kruse RW, Chen J. Accessory bones of the foot: clinical significance. Mil Med. 1995. 160:464–467.
3. Lawson JP, Ogden JA, Sella E, Barwick KW. The painful accessory navicular. Skeletal Radiol. 1984. 13:250–262.
4. Sella EJ, Lawson JP, Ogden JA. The accessory navicular synchondrosis. Clin Orthop. 1986. 209:280–285.
5. Miller TT, Staron RB, Feldman F, Parisien M, Glucksman WJ, Gandolfo LH. The symptomatic accessory tarsal navicular bone: assessment with MR imaging. Radiology. 1995. 195:849–853.
6. Demeyere N, De Maeseneer M, Osteaux M. Quiz case. Symptomatic type II accessory navicular. Eur J Radiol. 2001. 37:60–63.
7. Romanowski CA, Barrington NA. The accessory navicular-an important cause of medial foot pain. Clin Radiol. 1992. 46:261–264.
8. Mosel LD, Kat E, Voyvodic F. Imaging of the symptomatic type II accessory navicular bone. Australas Radiol. 2004. 48:267–271.
9. Shah S, Achong DM. The painful accessory navicular bone: scintigraphic and radiographic correlation. Clin Nucl Med. 1999. 24:125–126.
10. Grogan DP, Gasser SI, Ogden JA. The painful accessory navicular: a clinical and histopathological study. Foot Ankle. 1989. 10:164–169.
11. Sella EJ, Lawson JP. Biomechanics of the accessory navicular synchondrosis. Foot Ankle. 1987. 8:156–163.
12. Chen YJ, Hsu RW, Liang SC. Degeneration of the accessory navicular synchondrosis presenting as a rupture of the posterior tibial tendon. J Bone Joint Surg Am. 1997. 79:1791–1798.
13. Kidner FC. The prehallux in its relation to flat-foot. J Bone Joint Surg Am. 1929. 11:831.
14. Chater EH. Foot pain and the accessory navicular bone. Irish J Med Sci. 1962. 442:471–475.
15. Schweitzer ME, Caccese R. Karasick D, Wapner KL, Mitchell DG. Posterior tibial tendon tears: utility of secondary signs for MR-imaging diagnosis. Radiology. 1993. 188:655–659.
16. Chen YJ, Shih HN, Huang TJ, Hsu RW. Posterior tibial tendon tear combined with a fracture of the accessory navicular: a new subclassification? J Trauma. 1995. 39:993–996.
17. Kiter E, Erdag N, Karatosun V, Gunal I. Tibialis posterior tendon abnormalities in feet with accessory navicular bone and flatfoot. Acta Orthop Scand. 1999. 70:618–621.
18. Kiter E, Gunal I, Karatosun V, Korman E. The relationship between the tibialis posterior tendon and the accessory navicular. Ann Anat. 2000. 182:65–68.
19. Funk DA, Cass JR, Johnson KA. Acquired adult flatfoot secondary to posterior tibial-tendon pathology. J Bone Joint Surg Am. 1986. 68:95–102.
20. Rosenberg ZS, Cheung Y, Jahss MH, Noto AM, Norman A, Leeds NE. Rupture of the posterior tibial tendon: CT and MR imaging with surgical correlation. Radiology. 1988. 169:229–235.
21. Khoury NJ, el-Khoury GY, Saltzman CL, Brandser EA. MR imaging of posterior tibial tendon dysfunction. AJR Am J Roentgenol. 1996. 167:675–682.
22. Rosenberg ZS. Chronic rupture of the posteior tibial tendon. Magn Reson Imaging Clin N Am. 1994. 2:79–87.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download