Abstract
Objective
To compare conventional and diffusion-weighted MR imaging in terms of their depiction of the abnormalities occurring in Creutzfeldt-Jakob disease.
Materials and Methods
We retrospectively analyzed the findings of conventional (T2-weighted and fluid-attenuated inversion recovery) and diffusion-weighted MR imaging in four patients with biopsy-proven Creutzfeldt-Jakob disease. The signal intensity of the lesion was classified by visual assessment as markedly high, slightly high, or isointense, relative to normal brain parenchyma.
Results
Both conventional and diffusion-weighted MR images demonstrated bilateral high signal intensity in the basal ganglia in all four patients. Cortical lesions were observed on diffusion-weighted MR images in all four, and on fluid-attenuated inversion recovery MR images in one, but in no patient on T2-weighted images. Conventional MR images showed slightly high signal intensity in all lesions, while diffusion-weighted images showed markedly high signal intensity in most.
Creutzfeldt-Jakob disease (CJD) is a rare, transmissible illness that usually affects older adults and is characterized by rapidly progressive dementia, ataxia, myoclonus, and various other neurologic deficits such as visual disturbance (1). The infectious agent appears to consist of protein devoid of functional nucleic acid, which to distinguish it from viruses is termed a 'prion' (2). The diagnostic triad - progressive dementia, myoclonic jerks, and periodic sharp-wave EEG activity - may be lacking in as many as 25% of patients (1), and conventional magnetic resonance (MR) imaging revealed no abnormalities in 21% (3). It has recently been reported that diffusion-weighted MR imaging (DWI) in CJD patients may enhance premortem diagnostic accuracy (4-7). The present report compares the abnormalities seen on T2-weighted MR images, fluid-attenuated inversion recovery (FLAIR) MR images, and DWI in four patients with biopsy-proven CJD.
We reviewed conventional (T2-weighted and fluid-attenuated inversion recovery) and diffusion-weighted brain MR images in four patients [3 men, 1 woman; age range, 36-65 (mean, 54) years] in whom CJD was pathologically confirmed by brain biopsy. Histopathological examination of the biopsy specimen demonstrated spongiform degeneration, gliosis, and neuronal loss. Prion protein immunoreactivity was demonstrated in the biopsy specimen of all patients.
All MR images were obtained using a 1.5-T MRI Signa Horison Echospeed scanner (GE Medical Systems, Milwaukee, Wis., U.S.A.) or a Siemens Magnetom Vision Plus (Siemens, Erlangen, Germany). Conventional MR images were obtained with axial T2-weighted fast spin-echo sequences (TR/TE, 4000/98; three excitations), sagittal T1-weighted spin-echo sequences (TR/TE, 450/10; two excitations), axial contrast-enhanced (0.1 mmol/kg gadopentetate dimeglumine) T1-weighted spin-echo sequences, and axial FLAIR images (TR/TE, 9000/110 or 10002/126.5; TI, 2200 msec). DWI was performed in the axial plane using a single-shot spin-echo EPI sequence (TR/TE of 6500/110.1-137 ms, a 128×128 matrix, a 24×24 cm field of view, one excitation, and 5-mm slice thickness with a 1-3 mm gap). We used two b values (0 and 1000 sec/mm2) in two patients and three b values (0, 500, and 1000 sec/mm2) in the other two. Diffusion gradients were applied along three orthogonal directions (x-, y-, and z-axes).
MR images were analyzed qualitatively, focusing on the signal intensity of the lesions; on visual inspection, this was arbitrarily given one of three grades: markedly high, slightly high, or isointense, relative to normal brain parenchyma. In three patients, apparent diffusion coefficient (ADC) maps were created according to the Stejskal and Tanner formula (8). ADC values of each lesion were measured at the most conspicuous lesion in each cortex and basal ganglia by placing the regions-of-interest at the center of that lesion.
The MR imaging findings are summarized in Table 1. T2-weighted and FLAIR MR images showed slightly high signal intensity in the basal ganglia in all four patients (Figs. 1, 2, 3). Cortical lesions were, however, observed on FLAIR images in only one patient and in no patient on T2-weighted images. In contrast, DWI demonstrated increased signal intensity in both the basal ganglia and the cortex in all four patients. DWI showed a markedly high signal in 12 cortical regions and three basal ganglia, a slightly high signal in five cortical regions and one basal ganglia, and an isointense signal in three cortical regions. T1-weighted MR images obtained before and after gadopentetate dimeglumine administration were unremarkable except for diffuse atrophy.
The ADC values calculated in three patients were lower than those of normal brain parenchyma reported previously (9): values ranged from 0.42×10-3 to 0.65×10-3 mm2/s in lesions of the basal ganglia, and from 0.69×10-3 to 1.02×10-3 mm2/s in cortical lesions.
The clinical diagnosis of CJD is often difficult (1). Confirmatory diagnosis still relies on the results of brain biopsy or autopsy, with histological or biochemical examination. The demonstration of prion protein in brain tissue by immunohistochemical analysis represents the most specific and sensitive test to date for diagnosis of the condition.
In CJD, conventional MR images sometimes demonstrate an abnormally high signal on T2-weighted MR images of the basal ganglia (3). Although these findings are nonspecific, together with the clinical history they can help establish a diagnosis of probable CJD. Cortical lesions can be difficult to detect on T2-weighted MR images, though fluid attenuation sequences may increase the extent to which MR images can detect cortical abnormalities. An adjacent CSF signal may obscure abnormalities on T2-weighted MR images that are suppressed by FLAIR sequences.
There have been several reports of abnormal findings at DWI in patients with CJD (4-7). DWI has demonstrated a markedly increased signal in the basal ganglia and cortical regions, and in this respect is more sensitive than T2-weighted and FLAIR MR imaging. It has therefore been suggested that DWI may be useful for the early diagnosis of CJD. While conventional MR imaging has revealed discrete abnormalities only in the basal ganglia, DWI has disclosed multifocal regions of increased signal intensity in the cerebral cortices in addition to lesions of the basal ganglia. To our knowledge, diffusion abnormalities in both basal ganglia and cortices, with the same appearance as those reported here, have not been identified in other disease entities. Although anecdotal, these findings appear to be specific for CJD.
The characteristic neurohistopathologic features of CJD are limited to the central nervous system and are spongiform degeneration of the gray matter, which is characterized by individual and clustered vacuoles in neuronal and glial processes (10). Although the physicochemical basis for diffusion abnormalities in CJD remains unclear, it has been suggested that the cause of the observed diffusion restriction might be related to the presence of vacuoles seen histologically in spongiform degeneration (4-5). Diffusion of water molecules might be reduced owing to compartmentalization within the vacuoles. In addition, deposition of the prion protein might somehow restrict the free diffusion of water (6).
The normal mean ADC value reported by Gideon et al. (9) was 0.95×10-3 mm2/s in lentiform nuclei and 1.34×10-3 mm2/s in cortical gray matter. The ADC values reported in patients with CJD are variable. Several reports (4-6) have demonstrated that ADC values in lesions were significantly lower than in normal brain parenchyma, while ADC values of lesions in two patients of Memaerel et al. (7) were normal or mildly elevated. Agreeing with the former of these two opposing conclusions, our findings indicate the presence of restricted diffusion: the ADC values of lesions in our patients were much lower than those of normal gray matter. The abnormalities seen at DWI in some cortical lesions might, however, result from the synergistic effect of T2 shine-through and restriction of water diffusion in these lesions: their ADC values were nearly normal, and retrospective review of the T2-weighted images obtained disclosed subtle hyperintense signal in the same areas.
In variant CJD, so-called "mad cow disease", patients are younger, and psychiatric and sensory symptoms, which are frequently painful, are prominent at presentation (11). The important MR imaging feature in variant CJD is bilateral thalamic high signal; in particular, in the pulvinar, the so-called "pulvinar sign" (11). The thalamic changes occurring in variant CJD are symmetrical, in contrast to the changes in the basal ganglia occurring in sporadic CJD, which may be asymmetrical (11). Although the MR images in our case 4 showed asymmetrical involvement of the thalami, it is thought that variant CJD cannot be completely excluded.
In summary, DWI is more sensitive than conventional MR imaging in CJD. DWI appears to improve the in-vivo diagnosis of CJD and may thus reduce the number of false-negative results of MR examinations. In the absence of abnormalities on conventional MR images, DWI findings may help in guiding brain biopsy. To determine the specificity of these findings for CJD, further investigations must be performed.
Figures and Tables
Fig. 1
Case 1, a 63-year-old woman with Creutzfeldt-Jakob disease.
A and B. T2-weighted (A) and fluid-attenuated inversion recovery (B) MR images show bilateral high signal intensity in the putamen and caudate nucleus (arrows). There are small foci of high signal intensity in the frontal white matter (arrowhead), suggesting focal ischemia due to small vessel disease.
C. Diffusion-weighted MR image demonstrates bilateral high signal intensity in the frontal and insular cortices (arrows), as well as the basal ganglia.
D. Photomicrograph of the cerebral cortex shows numerous small vacuoles (arrows), indicating spongiform change and a reduced number of nerve cells (original magnification ×400; hematoxylin-eosin staining).
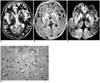
Fig. 2
Case 3, a 65-year-old man with Creutzfeldt-Jakob disease.
A. T2-weighted MR images show slightly increased signal intensity (arrows) in the putamen and caudate nucleus, bilaterally. High signal intensity foci (arrowheads) are seen in the posterior periventricular areas, suggesting focal ischemia due to small vessel disease.
B. Diffusion-weighted MR image demonstrates markedly increased signal intensity throughout the occipitotemporal cortex (arrows) and slightly increased signal intensity in the basal ganglia, bilaterally.
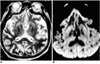
Fig. 3
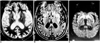
Case 4, a 36-year-old man with Creutzfeldt-Jakob disease.
A. T2-weighted MR image shows severe diffuse atrophy and slightly increased signal intensity in the basal ganglia, bilaterally (arrows).
B. Fluid-attenuated inversion recovery MR image shows bilaterally increased signal intensity in both the insular cortex (arrows) and basal ganglia.
C. Diffusion-weighted MR image demonstrates conspicuous bilateral high intensity in the insular cortex and basal ganglia (arrows). Subtle high signal intensity of the left thalamus (arrowhead) is also apparent.
References
1. Brown P, Cathala F, Castaigne P, Gajdusek DC. Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol. 1986. 20:597–602.
2. Prusiner SB. Molecular biology of prion diseases. Science. 1991. 252:1515–1522.
3. Finkenstaedt M, Szudra A, Zerr I, et al. MR imaging of Creutzfeldt-Jakob disease. Radiology. 1996. 199:793–798.
4. Bahn MM, Kido DK, Lin W, Pearlman AL. Brain magnetic resonance diffusion abnormalities in Creutzfeldt-Jakob disease. Arch Neurol. 1997. 54:1411–1415.
5. Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol. 1999. 56:577–583.
6. Na DL, Suh CK, Choi SH, et al. Diffusion-weighted magnetic resonance imaging in probable Creutzfeldt-Jakob disease. Arch Neurol. 1999. 56:951–957.
7. Demaerel P, Heiner L, Robberecht W, Sciot R, Wilms G. Diffusion-weighted MRI in sporadic Creutzfeldt-Jakob disease. Neurology. 1999. 52:205–208.
8. Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Physiol. 1965. 42:288–292.
9. Gideon P, Sorensen PS, Thomsen C, et al. Increased brain water self-diffusion in patients with idiopathic intracranial hypertension. AJNR. 1995. 16:381–387.
10. Masters CL, Richardson EP Jr. Subacute spongiform encephalopathy (Creutzfeldt-Jakob disease): the nature and progression of spongiform change. Brain. 1978. 101:333–344.
11. Zeidler M, Sellar RJ, Collie DA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet. 2000. 355:1412–1418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download