Abstract
Objective
To evaluate the incidence, types and association of systemic reactions after an epidural steroid injection (ESI) with patient demographics, ESI factors and repeated occurrence of an ESI.
Materials and Methods
This prospective observational study was approved by the Institutional Review Board of our hospital, and written informed consent was obtained from all the participants. From October to December 2011, systemic reactions at 2 weeks after 960 ESIs among 885 patients were measured. Patients were evaluated by phone interviews to obtain the patients' demographics, history of previous ESI, ESI factors, and ESI reoccurrence. Statistical analyses were performed using the chi-square tests, Fisher's exact tests and a binary logistic regression analysis.
Results
Overall, 557 types of systemic reactions occurred after 292 injections (30.4%) of a total of 960 ESIs in which facial flushing was most common (131/557, 23.5%) and 144 ESIs were followed by a mixed form of systemic reactions (49.3%). Age of 62 years or younger (odds ratio [OR], 2.361), female sex (OR, 1.674), and history of diabetes mellitus (OR, 1.681) were significant risk factors in the occurrence of systemic reactions after an ESI. In 73 patients with repeated ESI, 14 patients re-experienced systemic reactions (19.2%), of which twelve re-experienced the same systemic reaction as the previous one.
Conclusion
Systemic reactions followed about 30% of ESIs, and more commonly occurred in patients 62 years of age or younger, women, and diabetic patients. Half of the patients experienced a mixed form of systemic reactions. Patients with recurring systemic reactions tend to re-experience the same systemic reaction as the prior one after an ESI.
Epidural steroid injections (ESIs) have become increasingly common for the treatment of a variety of pain, including low back pain (123), and its effectiveness has been confirmed by many studies (4567). However, various systemic reactions associated with ESIs have also been reported, including vasovagal reactions, allergic reactions, headaches, facial flushing, gastrointestinal disorders, cardiovascular disorders, weight/appetite change, and psychiatric issues (891011121314151617181920).
Several studies have investigated the incidence and types of systemic reactions associated with ESI; however, in clinical practice, the results of these studies cannot be applied as guidelines regarding the risk factors or prevention of ESI-related systemic reactions for the following reasons (891011121314151617181920): 1) a majority of them were retrospective studies based on chart reviews; 2) different types and doses of steroid drugs were used; 3) duration between the ESI and follow-up varied between the studies; and, 4) the occurrence of systemic or local adverse reactions was not investigated in terms of its relationship to the number of reoccurences of ESI, patients' demographics such as age and sex, and underlying diseases such as diabetic mellitus or hypertension.
Therefore, we conducted this prospective observational study to determine the incidence and types of systemic reactions occurring after an ESI in a large population, and to evaluate their association with factors such as patients' demographics (age, sex, and underlying disease), ESI factors (injection method, injection site, type and dose of steroid, and injection practitioner) and recurrence of an ESI.
This prospective observational study was approved by the Institutional Review Board of our hospital, and written informed consent was obtained from all the participants. This study was originally designed to have 1000 ESIs, according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (21). This study was registered with the Clinical Trials Registry (NCT01756196).
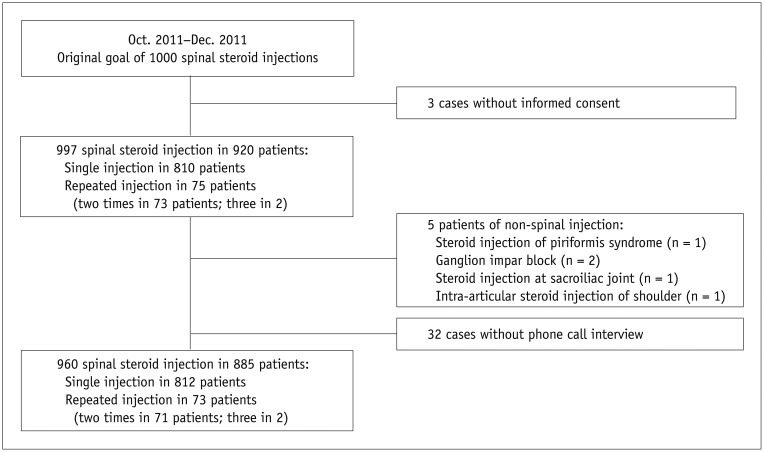
All patients were referred by clinicians for an injection treatment for low back pain, cervical pain, or radiculopathy. All injection treatments were performed after an interview by two professors of spine pain intervention (with 13 and 7 years of experiences in spine pain intervention) at our institute from October to December 2011. Patients with persistent pain despite conservative treatment (e.g., oral analgesics), who showed 5 points or more on the visual analog scale, whose suspected cause of their pain was documented on CTs or MRIs (e.g. disc herniation or spinal stenosis), and who had no history of absolute/relative contraindications of ESI (e.g., uncorrected coagulopathy, pregnancy, suspected infection) were included. All patients that received an injection were informed of the aim and protocol of this study as well as the possible systemic reactions associated with an ESI. We recorded underlying diseases such as diabetes mellitus and hypertension, anticoagulant medication, and history of previous ESIs. After excluding 3 patients who denied consent and 5 patients who received non-ESI treatment (i.e., one piriformis injection; 2 ganglion impar blocks; one sacroiliac joint injection; and one shoulder injection) from the scheduled 1000 injection cases, 992 ESIs were performed in 915 patients: a single injection in 840 patients, a double ESI in 73 patients, and a triple ESI in two patients. After excluding 32 cases who did not participate in phone interviews (32/992, drop rate of injections = 3.2%), 960 ESIs in 885 patients were finally included in this study (Fig. 1). Baseline demographics of the 885 patients are listed in Table 1.
In this study, various methods and drugs were used for ESIs, and combination of injection methods and injection sites were permitted during each ESI (Table 2). All procedures were performed using 22- or 25-gauge spinal needles under fluoroscopic guidance by one of four radiologists (13 years, 7 years, and 2 with one year of experience in spine pain interventions). Triamcinolone acetonide (Triam 40 mg/mL; Shin Poong Pharm, Seoul, Korea) was used for all procedures except for the transforaminal epidural injections to the cervical spine, which was performed using Dexamethasone Disodium Phosphate (5 mg/mL; Huons, Seongnam, Korea). The total daily dose of steroid was limited to 40 mg of triamcinolone acetonide or 10 mg of dexamethasone disodium phosphate; doses for multiple ESIs on a single day were calculated accordingly. For diabetic patients, the steroid dose was halved (i.e., 20 mg of triamcinolone acetonide). We used 25-gauge instead of the usual 22-gauge spinal needles in 127 patients on oral anticoagulant medication (142 ESI) after the discontinuation of medication for an appropriate period of time according to the drug.
All patients were scheduled to receive a phone call 2 weeks after each ESI. The mean time interval between the ESI and phone call was 15 days (range, 12–25 days). All phone interviews were conducted by an expert nurse with 2 years of experience in the outpatient clinic for spinal intervention. During the phone interview, patients were asked to report any systemic reactions after the ESI. The list of systemic reactions included facial flushing, itching or allergic reaction, headache, cardiovascular disorders (chest pain, palpitation, increased blood pressure, etc.), night sweats, fever or chilling sense, menstrual change (cyclic irregularity, menorrhagia, etc.), erectile dysfunction, urination difficulty, increased blood glucose level, gastrointestinal disorders (nausea, vomiting, diarrhea, constipation, incontinence, etc.), psychiatric issues (insomnia, psychosis, etc.), weight change, change in appetite, general weakness, dizziness, polyuria, thirst, tinnitus, hiccups, eye problems (blood-shot eye, blurred vision, etc.), and hair loss.
The occurrence of systemic reactions after an ESI was investigated by the chi-square test or Fisher's exact test according to the patients' demographics (i.e., history of hypertension or diabetes mellitus, anticoagulant medication, age, and sex), and factors associated with ESIs (i.e., injection number, injection site, method, dose and type of steroid, and injection practitioner). Binary logistic regression analysis was used to estimate the risk of systemic reactions based on patients' baseline demographics, defined as exp(B) (odds ratio, OR). Sub-group analyses were also conducted for the patients with diabetes mellitus, hypertension, and reccurring ESI. A p value of less than 0.05 was considered significant. All statistical analyses were performed using a statistical software (PASW, version 17.0; SPSS Inc., Chicago, IL, USA).
Overall, 557 types of systemic reactions occurred after 292 injections out of a total of 960 ESIs (292/960, 30.4%) in 271 patients (271/885, 30.6%) (Table 3). In 292 ESIs with systemic reactions, 148 exhibited only one type of systemic reaction (148/292, 50.7%) among 148 patients (148/885, 16.7%); whereas, 144 ESIs were followed by two types or more (mixed form) of systemic reactions (144/292, 49.3%) among 134 patients (134/885, 15.1%). Among the 557 types of systemic reactions, facial flushing (131/557, 23.5%; 122/885 patients, 13.8%), weight/appetite changes (67/557, 12.0%; 65/885 patients, 7.3%), gastrointestinal problems (57/557, 10.2%; 54/885 patients, 6.1%) and cardiovascular problems (55/557, 9.8%; 52/885 patients, 5.9%) were encountered. These systemic reactions improved spontaneously, and no major complications that required hospitalization or intensive medical treatment was reported.
Based on the patients' mean age (62 years), the overall incidence of systemic reaction was significantly more common in patients of 62 years of age or younger (160/389 patients, 41.1%) than in patients who were over 62 years of age (121/496 patients, 24.4%; p < 0.001). With respect to sex, systemic reactions were more frequently present in women (193/547, 35.3%) than in men (88/338, 26.0%; p = 0.005). However, a history of hypertension, diabetes mellitus, or anticoagulant medication was not significantly related to the overall incidence of systemic reactions (p > 0.05). A calculation of the risk estimation using binary logistic regression revealted that an age of 62 years or younger (OR, 2.361), female sex (OR, 1.674), and history of diabetes mellitus (OR, 1.681) were significant risk factors in the occurrence of systemic reactions after an ESI (Table 4).
The method, site, type and dose of steroid, and injection practitioner of an ESI were not associated with the occurrence of systemic reactions (p > 0.05). The number of previous ESIs and the time interval between ESIs were not related with the incidence of systemic reactions (p > 0.05).
Among 130 ESIs among 118 diabetic patients, 89 types of systemic reaction occurred after 49 ESIs (49/130, 37.7%) in 46 patients (46/118, 39.0%) and an increased blood glucose level was the most frequent symptom (27/89, 30.3%; 27/46 patients, 58.7%). Only a half dose of steroid was supposed to be injected for diabetic patients; however, fifteen patients (11.5%, 15/118 patients) had an ESI using a full-dose of steroid (i.e., triamcinolone 40 mg) because knowledge of the presence of diabetes mellitus did not occur until after the ESI versus during patient enrollment when patients' histories were obtained. Facial flushing (12/89, 13.5%; 11/46 patients, 23.9%), constipation (8/89, 9.0%; 8/46 patients, 17.4%), and psychiatric problems (9/89, 10.1%; 9/46 patients, 19.6%) were also common in diabetic patients (Table 5).
In 75 of the 233 hypertensive patients (75/233, 32.2%), 152 types of systemic reaction occurred in 84 ESIs out of 257 ESIs (84/257, 32.7%). Facial flushing was the most frequent symptom (30/152, 19.7%; 28/75 patients, 37.3%), followed by cardiovascular problems (21/152, 13.8%, 14/75 patients, 18.7%). In addition, weight/appetite change (15/152, 9.9%; 15/75 patients, 20.0%), night sweats (14/152, 9.2%; 14/75 patients, 18.7%), headaches (12/152, 7.9%; 12/75 patients, 16.0%), and fever/chilling sense (10/152, 6.6%; 10/75 patients, 13.3%) were common (Table 5).
In 73 patients with two or more ESIs during the study period (mean time interval between ESIs, 39.6 days; range, 7–176 days), 14 patients re-experienced systemic reactions (14/73, 19.2%). Twelve of those 14 patients had the same form of systemic reaction as the previous ESI and two had mixed forms with the same and different systemic reactions. Facial flushing was the most common reaction and occurred in 7 patients (7/14, 50%). Systemic reactions after repeated ESIs are presented in Table 6.
Epidural steroid injections have been used worldwide for the conservative management of spinal pain, and its effectiveness and safety has been widely reported (1234567). However, many studies have reported a wide range of complications such as minor or major local or systemic reactions (22232425262728293031323334353637383940). In clinical practice, it is very important for practitioners to understand the possible complications of ESI in order to prevent and manage them.
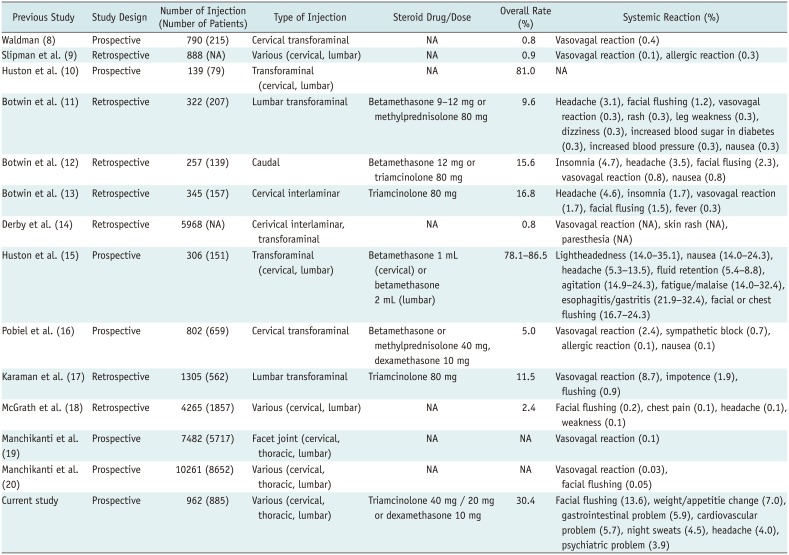
Previous studies have reported a very wide range of overall incidence of adverse reactions: 0.8–86.5% with 0.08–12.1% representing systemic reactions, due to the heterogeneity of the study designs (Table 7). In this study, the overall incidence of systemic reactions was relatively high at 30.4%, compared to several previous studies (89181920). Only one study revealed a high incidence of systemic reactions with a significant difference between the cervical and lumbar ESIs; however, it did not evaluate the relationship with the underlying disease (15). In contrast, our study revealed a significantly high risk of increased blood sugar levels in diabetic patients. Another study demonstrated a significant difference in the incidence of adverse reactions between interlaminar (6.0%) and transforaminal (2.1%) ESIs (18). In our study, however, no significant difference according to the site (cervical vs. lumbar spine) (p = 0.057) or the method of ESI (p = 0.590) was noted. In our study, there was also no difference according to the injection practitioners (p = 0.789), which could not be compared to other studies because that comparison has not been studied previously.
It may be difficult to perform a direct comparison between the results of previous studies and those of our study because in several of the previous studies, the systemic reactions are not explained in detail. These studies were focused on the immediate complications after an ESI, with little interest in the patients' demographics such as an underlying disease or the number of previous ESIs (891011121314151617181920). Botwin et al. (12) demonstrated that adverse reactions were more commonly witnessed in patients older than 70 years; however, another study by Botwin et al. (13) showed no differences in adverse reactions according to the age and history of a prevous ESI. In our study, systemic reactions occurred more frequently in patients 62 years of age or younger which suggest that this population has more significiant risk (OR, 2.361).
Botwin et al. (11) reported no mixed forms of systemic reactions; whereas, in our study, about half of the ESIs that were followed by a systemic reaction had mixed forms consisting of two types of systemic reactions or more (144/292, 49.3%). This result may indicate that patients who are susceptible to one adverse response after an ESI may suffer from a wide range of complications. On the other hand, no difference in incidence of systemic reactions according to the number of previous ESIs during the previous 6 months was noted (p = 0.940). This suggests that systemic reactions do not occur as a result of a cumulative effect of ESIs.
In this study, after a repeated ESI, systemic reactions recurred in 14 patients (14/73), among whom 12 had the same form as the occurrence after a previous ESI. Thus, this implies that the pattern of recurrent systemic reactions may be similar.
In this study, an increased blood glucose level was the most frequent systemic reaction in diabetic patients. In a previous study in which glucose tolerance was evaluated in only 5 diabetic patients after a lumbar ESI using 80 mg of methylprednisolone, no effect was reported on the glycemic control in diabetic patients (41). At the same time, two recent studies demonstrated a significant ephemeral increase in blood glucose levels after an ESI in the lumbar spine using betamethasone (i.e., a variable dose according to the injection method in 1 patient; 40 mg betamethasone in the other patients), which are similar to the results of this study (4243). However, we did not obtain serial measurements of the glucose level after ESIs and we used a different steroid (triamcinolone, 40 mg) in both non-diabetic and diabetic patients. The increased blood glucose levels were entirely dependant on the diabetic patients' self-check conducted at home. So, the results of this study regarding increased blolod glucose level must be interpreted and adopted in clinical practice with caution. Nevertheless, the occurrence of many diabetic patients experiencing transient but abnormal and abrupt increases in blood glucose levels in our study may suggest a risk or trend of transient but abrupt increases in blood glucose levels among diabetic patients after an ESI.
Cardiovascular symptoms were the second most common reaction in hypertensive patients. This outcome could not be compared to previous studies because hypertensive patients have not yet been evaluated in this regard. However, it was impossible to measure blood pressure objectly in both of the normotensive and hypertensive patients and adjust the variables such as the method or timing of blood pressure measure or the use of antihypertensive druga. Thus, our results may solely raise a possibility of a transient increase in blood pressure in hypertensive patients after an ESI.
Our study has the following limitations. First, systemic reactions after ESIs were investigated after only a short period of time (i.e., 2 weeks) after the ESI; so, long-term follow-up results could not be evaluated. Second, follow-up measurements of blood glucose levels were not performed in either non-diabetic nor diabetic patients and only was evaluated by the patients' self-report of blood glucose test results. Therefore, it was not possible to calculate the degree of increase in blood glucose levels as a percentage objectively. In addition, we failed to adjust for the effects of hypoglycemic agents and injected steroid doses which could influence the results related to blood glucose levels. Likewise, the follow-up measurements of blood pressure were also not obtained in both normotensive and hypertensive patients. Moreover, considering many factors influence blood pressure, such as the timing of and method for blood pressure measurement or antihypertensive drugs, the increase in blood pressure due to an ESI may be subjective or inaccurate in our study. Nevertheless, in this study, many diabetic patients complained or worried about an increase in blood glucose levels and many hypertensive patients were nervous also about an increase in blood pressure after an ESI. Therefore, our results suggests a possible risk related to ESIs.
In conclusion, systemic reactions occur in about 30% of ESIs, especially among patients that are 62 years of age or younger, women, and diabetic patients. Approximately half of the patients will experience a mixed form of systemic reactions. Furthermore, for those experience recurrence of an ESI, the same systemic reaction may result as previously occurred after an ESI.
References
1. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009; 22:62–68. PMID: 19124635.

2. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976). 2013; 38:157–168. PMID: 22781007.
3. Manchikanti L, Singh V, Pampati V, Smith HS, Hirsch JA. Analysis of growth of interventional techniques in managing chronic pain in the Medicare population: a 10-year evaluation from 1997 to 2006. Pain Physician. 2009; 12:9–34. PMID: 19165296.
4. Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009; 12:233–251. PMID: 19165306.
5. Conn A, Buenaventura RM, Datta S, Abdi S, Diwan S. Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2009; 12:109–135. PMID: 19165299.
6. Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007; 10:185–212. PMID: 17256030.
7. Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007; 10:229–253. PMID: 17256032.
8. Waldman SD. Complications of cervical epidural nerve blocks with steroids: a prospective study of 790 consecutive blocks. Reg Anesth. 1989; 14:149–151. PMID: 2486595.
9. Slipman CW, Meyers JS, Chou LH, Sterenfeld EB, Abrams S. Complications of fluoroscopically guided spinal injections (abstract). Arch Phys Med Rehabil. 1995; 76:1032.
10. Huston CW, Slipman CW, Meyers JS, Yang ST, Anghel BN. Side effects and complications of fluoroscopically guided nerve root injections (abstract). Arch Phys Med Rehabil. 1996; 77:937.
11. Botwin KP, Gruber RD, Bouchlas CG, Torres-Ramos FM, Freeman TL, Slaten WK. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2000; 81:1045–1050. PMID: 10943753.

12. Botwin KP, Gruber RD, Bouchlas CG, Torres-Ramos FM, Hanna A, Rittenberg J, et al. Complications of fluoroscopically guided caudal epidural injections. Am J Phys Med Rehabil. 2001; 80:416–424. PMID: 11399002.

13. Botwin KP, Castellanos R, Rao S, Hanna AF, Torres-Ramos FM, Gruber RD, et al. Complications of fluoroscopically guided interlaminar cervical epidural injections. Arch Phys Med Rehabil. 2003; 84:627–633. PMID: 12736872.

14. Derby R, Lee SH, Kim BJ, Chen Y, Seo KS. Complications following cervical epidural steroid injections by expert interventionalists in 2003. Pain Physician. 2004; 7:445–449. PMID: 16858486.
15. Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005; 86:277–283. PMID: 15706554.

16. Pobiel RS, Schellhas KP, Eklund JA, Golden MJ, Johnson BA, Chopra S, et al. Selective cervical nerve root blockade: prospective study of immediate and longer term complications. AJNR Am J Neuroradiol. 2009; 30:507–511. PMID: 19193762.

17. Karaman H, Kavak GO, Tüfek A, Yldrm ZB. The complications of transforaminal lumbar epidural steroid injections. Spine (Phila Pa 1976). 2011; 36:E819–E824. PMID: 21217425.

18. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011; 12:726–731. PMID: 21392252.

19. Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. Complications of fluoroscopically directed facet joint nerve blocks: a prospective evaluation of 7,500 episodes with 43,000 nerve blocks. Pain Physician. 2012; 15:E143–E150. PMID: 22430660.
20. Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician. 2012; 15:131–140. PMID: 22430650.
21. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147:573–577. PMID: 17938396.

22. Simopoulos TT, Kraemer JJ, Glazer P, Bajwa ZH. Vertebral osteomyelitis: a potentially catastrophic outcome after lumbar epidural steroid injection. Pain Physician. 2008; 11:693–697. PMID: 18850035.
23. Ain RJ, Vance MB. Epidural hematoma after epidural steroid injection in a patient withholding enoxaparin per guidelines. Anesthesiology. 2005; 102:701–703.

24. Xu R, Bydon M, Gokaslan ZL, Wolinsky JP, Witham TF, Bydon A. Epidural steroid injection resulting in epidural hematoma in a patient despite strict adherence to anticoagulation guidelines. J Neurosurg Spine. 2009; 11:358–364. PMID: 19769520.

25. MacLean CA, Bachman DT. Documented arterial gas embolism after spinal epidural injection. Ann Emerg Med. 2001; 38:592–595. PMID: 11679875.

26. Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine (Phila Pa 1976). 2005; 30:E266–E268. PMID: 15897816.
27. Muro K, O'Shaughnessy B, Ganju A. Infarction of the cervical spinal cord following multilevel transforaminal epidural steroid injection: case report and review of the literature. J Spinal Cord Med. 2007; 30:385–388. PMID: 17853663.

28. Suresh S, Berman J, Connell DA. Cerebellar and brainstem infarction as a complication of CT-guided transforaminal cervical nerve root block. Skeletal Radiol. 2007; 36:449–452. PMID: 17216270.

29. Lyders EM, Morris PP. A case of spinal cord infarction following lumbar transforaminal epidural steroid injection: MR imaging and angiographic findings. AJNR Am J Neuroradiol. 2009; 30:1691–1693. PMID: 19369604.

30. Popescu A, Lai D, Lu A, Gardner K. Stroke following epidural injections--case report and review of literature. J Neuroimaging. 2013; 23:118–121. PMID: 21699610.

31. Kushner FH, Olson JC. Retinal hemorrhage as a consequence of epidural steroid injection. Arch Ophthalmol. 1995; 113:309–313. PMID: 7887844.

32. Young WF. Transient blindness after lumbar epidural steroid injection: a case report and literature review. Spine (Phila Pa 1976). 2002; 27:E476–E477. PMID: 12439000.
33. Browning DJ. Acute retinal necrosis following epidural steroid injections. Am J Ophthalmol. 2003; 136:192–194. PMID: 12834695.

34. Bhat AL, Chow DW, DePalma MJ, Garvan C, Chou L, Lenrow D, et al. Incidence of vocal cord dysfunction after fluoroscopically guided steroid injections in the axial skeleton. Arch Phys Med Rehabil. 2005; 86:1330–1332. PMID: 16003659.

35. Everett CR, Baskin MN, Speech D, Novoseletsky D, Patel R. Flushing as a side effect following lumbar transforaminal epidural steroid injection. Pain Physician. 2004; 7:427–429. PMID: 16858483.
36. Abbasi A, Roque-Dang CM, Malhotra G. Persistent hiccups after interventional pain procedures: a case series and review. PM R. 2012; 4:144–151. PMID: 22373464.

37. Benyamin RM, Vallejo R, Kramer J, Rafeyan R. Corticosteroid induced psychosis in the pain management setting. Pain Physician. 2008; 11:917–920. PMID: 19057637.
38. Sandberg DI, Lavyne MH. Symptomatic spinal epidural lipomatosis after local epidural corticosteroid injections: case report. Neurosurgery. 1999; 45:162–165. PMID: 10414580.

39. Goodman BS, Bayazitoglu M, Mallempati S, Noble BR, Geffen JF. Dural puncture and subdural injection: a complication of lumbar transforaminal epidural injections. Pain Physician. 2007; 10:697–705. PMID: 17876368.
40. Candido KD, Katz JA, Chinthagada M, McCarthy RA, Knezevic NN. Incidence of intradiscal injection during lumbar fluoroscopically guided transforaminal and interlaminar epidural steroid injections. Anesth Analg. 2010; 110:1464–1467. PMID: 20418306.

41. Zufferey P, Bulliard C, Gremion G, Saugy M, So A. Systemic effects of epidural methylprednisolone injection on glucose tolerance in diabetic patients. BMC Res Notes. 2011; 4:552. PMID: 22185681.

42. Gonzalez P, Laker SR, Sullivan W, Harwood JE, Akuthota V. The effects of epidural betamethasone on blood glucose in patients with diabetes mellitus. PM R. 2009; 1:340–345. PMID: 19627917.

43. Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural steroid injections on blood glucose levels in patients with diabetes mellitus. Spine (Phila Pa 1976). 2012; 37:E46–E50. PMID: 21540770.

Table 1
Baseline Demographics of Patients
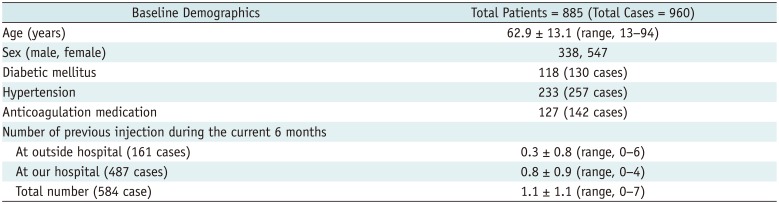
Table 2
Factors Associated with Epidural Steroid Injection
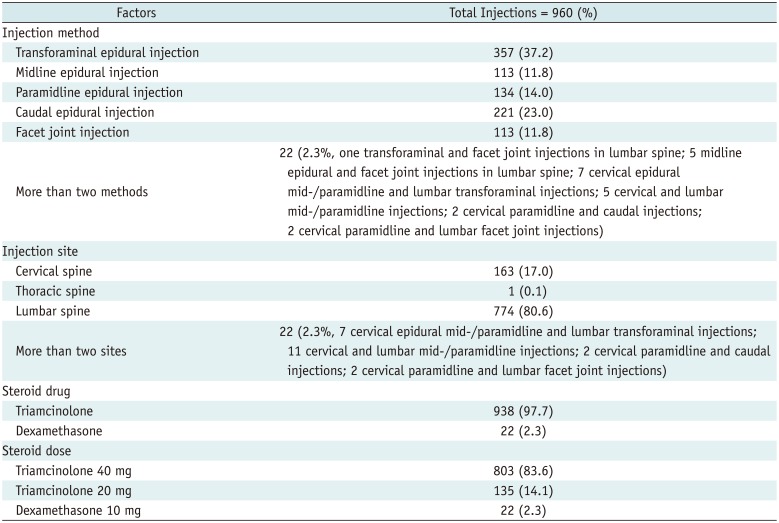
Table 3
Systemic Reactions after Total 960 Epidural Steroid Injections
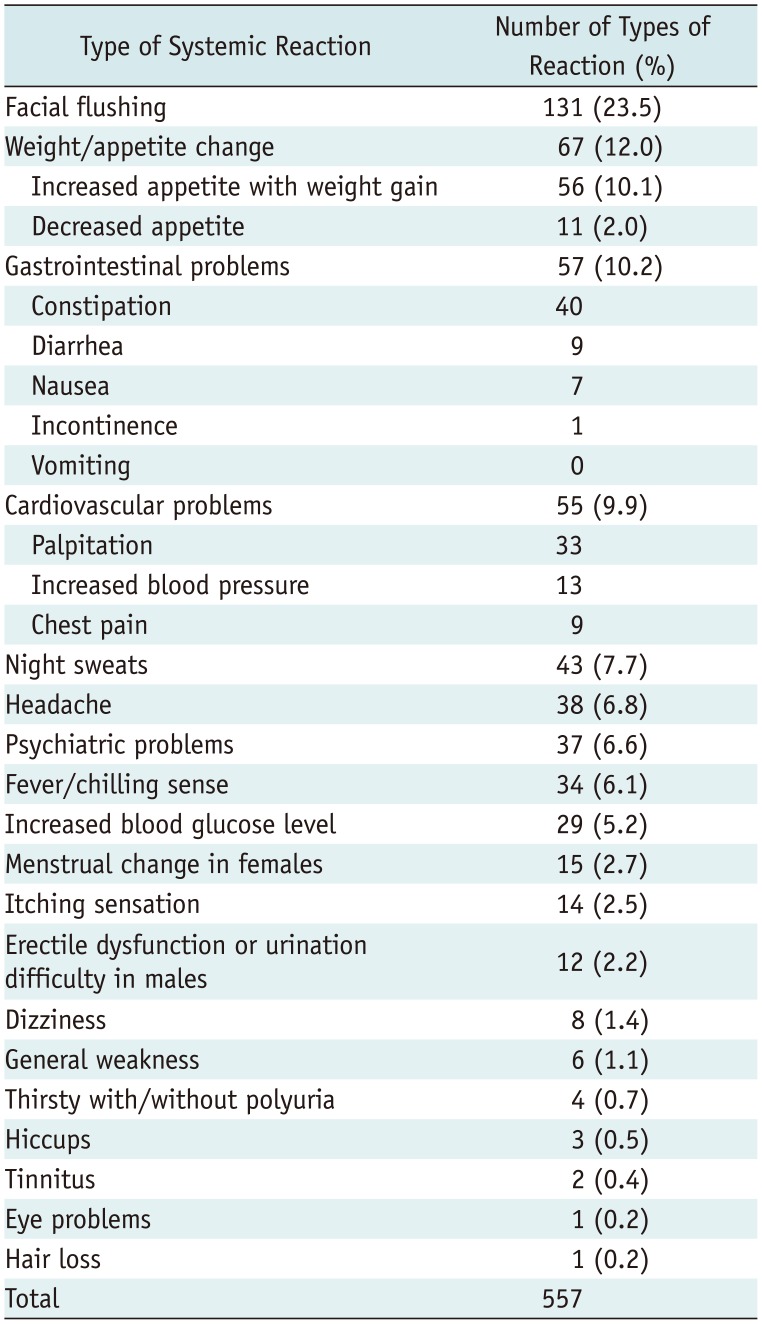
Table 4
Risk Estimation for Systemic Reactions in Total 960 Epidural Steroid Injections
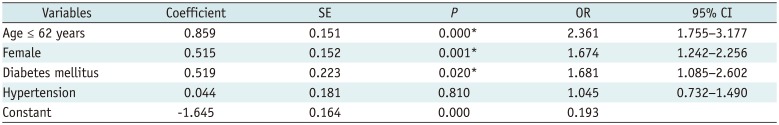
Table 5
Systemic Reactions in Diabetic and/or Hypertensive Patients
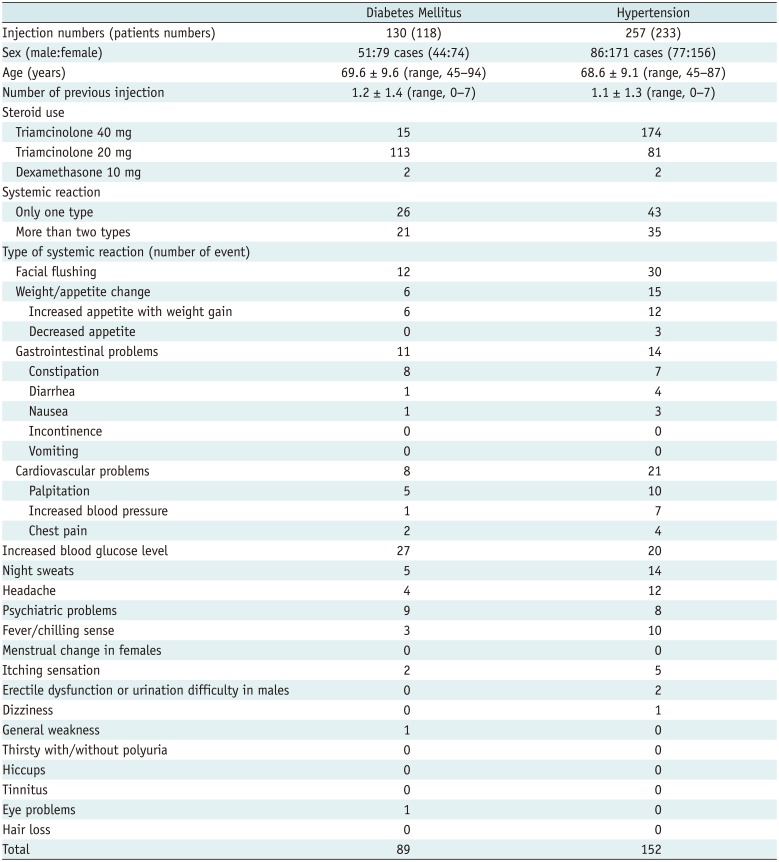
Table 6
Systemic Reactions after Repeated Epidural Steroid Injection in 73 Patients (148 Injections)
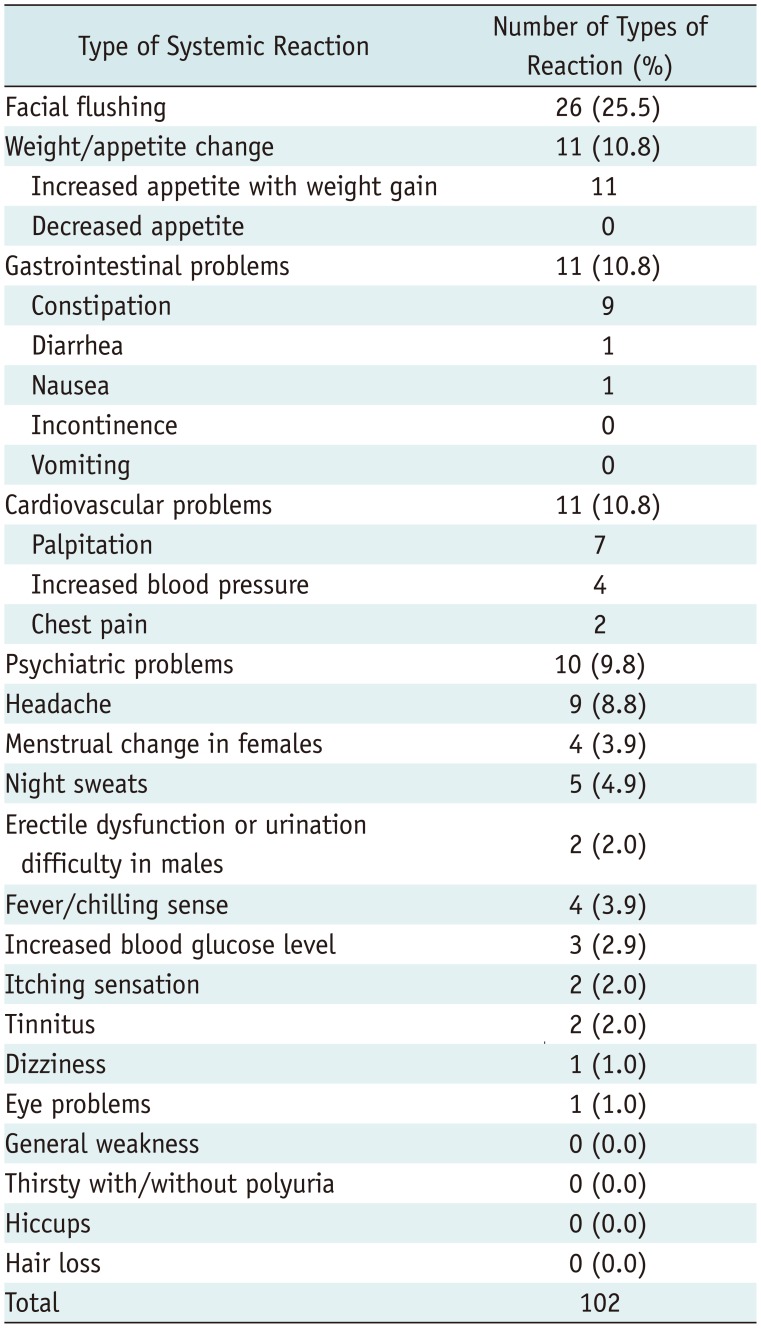
Table 7
Comparison of Current Study with Previous Studies about Systemic Reactions after Epidural Steroid Injection
| Previous Study | Study Design | Number of Injection (Number of Patients) | Type of Injection | Steroid Drug/Dose | Overall Rate (%) | Systemic Reaction (%) |
|---|---|---|---|---|---|---|
| Waldman (8) | Prospective | 790 (215) | Cervical transforaminal | NA | 0.8 | Vasovagal reaction (0.4) |
| Slipman et al. (9) | Retrospective | 888 (NA) | Various (cervical, lumbar) | NA | 0.9 | Vasovagal reaction (0.1), allergic reaction (0.3) |
| Huston et al. (10) | Prospective | 139 (79) | Transforaminal (cervical, lumbar) | NA | 81.0 | NA |
| Botwin et al. (11) | Retrospective | 322 (207) | Lumbar transforaminal | Betamethasone 9–12 mg or methylprednisolone 80 mg | 9.6 | Headache (3.1), facial flushing (1.2), vasovagal reaction (0.3), rash (0.3), leg weakness (0.3), dizziness (0.3), increased blood sugar in diabetes (0.3), increased blood pressure (0.3), nausea (0.3) |
| Botwin et al. (12) | Retrospective | 257 (139) | Caudal | Betamethasone 12 mg or triamcinolone 80 mg | 15.6 | Insomnia (4.7), headache (3.5), facial flusing (2.3), vasovagal reaction (0.8), nausea (0.8) |
| Botwin et al. (13) | Retrospective | 345 (157) | Cervical interlaminar | Triamcinolone 80 mg | 16.8 | Headache (4.6), insomnia (1.7), vasovagal reaction (1.7), facial flusing (1.5), fever (0.3) |
| Derby et al. (14) | Retrospective | 5968 (NA) | Cerivical interlaminar, transforaminal | NA | 0.8 | Vasovagal reaction (NA), skin rash (NA), paresthesia (NA) |
| Huston et al. (15) | Prospective | 306 (151) | Transforaminal (cervical, lumbar) | Betamethasone 1 mL (cervical) or betamethasone 2 mL (lumbar) | 78.1–86.5 | Lightheadedness (14.0–35.1), nausea (14.0–24.3), headache (5.3–13.5), fluid retention (5.4–8.8), agitation (14.9–24.3), fatigue/malaise (14.0–32.4), esophagitis/gastritis (21.9–32.4), facial or chest flushing (16.7–24.3) |
| Pobiel et al. (16) | Prospective | 802 (659) | Cervical transforaminal | Betamethasone or methylprednisolone 40 mg, dexamethasone 10 mg | 5.0 | Vasovagal reaction (2.4), sympathetic block (0.7), allergic reaction (0.1), nausea (0.1) |
| Karaman et al. (17) | Retrospective | 1305 (562) | Lumbar transforaminal | Triamcinolone 80 mg | 11.5 | Vasovagal reaction (8.7), impotence (1.9), flushing (0.9) |
| McGrath et al. (18) | Retrospective | 4265 (1857) | Various (cervical, lumbar) | NA | 2.4 | Facial flushing (0.2), chest pain (0.1), headache (0.1), weakness (0.1) |
| Manchikanti et al. (19) | Prospective | 7482 (5717) | Facet joint (cervical, thoracic, lumbar) | NA | NA | Vasovagal reaction (0.1) |
| Manchikanti et al. (20) | Prospective | 10261 (8652) | Various (cervical, thoracic, lumbar) | NA | NA | Vasovagal reaction (0.03), facial flushing (0.05) |
| Current study | Prospective | 962 (885) | Various (cervical, thoracic, lumbar) | Triamcinolone 40 mg / 20 mg or dexamethasone 10 mg | 30.4 | Facial flushing (13.6), weight/appetitie change (7.0), gastrointestinal problem (5.9), cardiovascular problem (5.7), night sweats (4.5), headache (4.0), psychiatric problem (3.9) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download