Abstract
Objective
To evaluate the feasibility and image quality (IQ) of prospectively high-pitch coronary CT angiography (CCTA) with low contrast medium injection rate at 70 kVp.
Materials and Methods
One hundred and four patients with suspected coronary artery disease (body mass index < 26 kg/m2, sinus rhythm and heart rate < 70 beats/min) were prospectively enrolled and randomly divided into two groups. In group A and group B, 28 mL and 40 mL of 370 mgI/mL iodinated contrast media was administrated at a flow rate of 3.5 and 5 mL/s, respectively. CT values, noise, signal-to-noise ratio, contrast-to-noise ratio (CNR) of the proximal segments of coronary arteries and subjective IQ were evaluated.
Results
The CT values and noise in group A were significantly lower than those in group B (434–485 Hounsfield units [HU] vs. 772–851 HU, all p < 0.001; 17.8–22.3 vs. 23.3–26.4, all p < 0.005). The CNRs of the right coronary artery and left main artery showed no statistical difference between the two groups (42.1 ± 13.8 vs. 36.8 ± 16.0, p = 0.074; 38.7 ± 10.6 vs. 38.1 ± 17.0, p = 0.819). No statistical difference was observed between the two groups in IQ scores (3.04 ± 0.75 vs. 3.0 ± 0.79, p = 0.526) and diagnostic ratio (96.1% [50/52] vs. 94.2% [49/52], p = 0.647).
Coronary artery angiography has become the preferred method for coronary artery disease screening and diagnosis as a non-invasive technique (1234). However, high radiation dose and the amount of required contrast medium limit its widespread clinical application (56).
Recently, a variety of dose reduction methods have been reported including enhancing the detector sensitivity (7), reducing the tube voltage (8910), high-pitch scan mode (1112) and iterative image reconstruction (13). Of these techniques, 70 kVp scanning could significantly reduce the radiation dose. In Zhang's study (14), the effective dose (ED) was reduced to 0.2 mSv, when 70 kVp scanning was adopted in coronary CT angiography (CCTA) for patients with body mass index (BMI) < 25 kg/m2. In addition, 70 kVp scanning could largely enhance the image contrast which could consequently decrease the contrast media volume and reduce risk for contrast-induced nephropathy (1516). Nevertheless, 70 kVp scanning has some limitations, which include an increase in image noise and compromise in image quality (IQ). To achieve diagnostic IQ, higher tube current or iterative reconstruction technique is required to compensate for the IQ degradation caused by lowering tube voltage (1718). To our best knowledge, no study has been conducted on coronary artery CT angiography (CTA) at 70 kVp with low contrast media injection rate.
Herein, we aimed to evaluate the feasibility and IQ of prospectively high-pitch CCTA with low contrast medium injection rate (3.5 mL/s) and low contrast medium amount (28 mL) at 70 kVp by comparing with the routine contrast medium injection protocol.
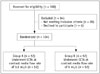
This study was approved by the local Institutional Review Board and informed consent was obtained from all patients before coronary artery CTA examinations. We assessed 188 patients indicated for coronary artery CTA; and finally, a total of 104 eligible patients were enrolled in the study (Fig. 1). Patients with age > 18 years, BMI < 26 kg/m2, and sinus rhythm and heart rate (HR) ≤ 70 beats per minute (bpm) were included. Exclusion criteria included patients with HR > 70 bpm, arrhythmia with heart rate variation (HRV; defined as the difference between minimum and maximum HR in the 10 heartbeats before imaging acquisition) > 20 bpm, known or suspected iodine contrast media allergy and hyperthyroidism. Patients with renal insufficiency (creatinine level ≥ 120 µmol/L), pregnant women and hemodynamically unstable patients were also excluded from the study.
The clinical indications of 104 patients for coronary artery CTA included: 1) suspected coronary heart disease with symptoms of atypical chest pain or shortness of breath (n = 20); 2) screening for medium or high risk population without symptom (medium or high risk population refers to patients with at least 2 risk factors of coronary heart disease: such as sex, age, positive family history, hypertension [defined as systolic blood pressure ≥ 140 mm Hg], diabetes, hyperlipoidemia, smoking, etc.) (n = 57); 3) follow-up of treated patients with known coronary heart disease or coronary atherosclerotic plaques (n = 28); and 4) preoperative evaluation of percutaneous coronary intervention (n = 3).
Included patients (n = 104) were randomly assigned into the experimental group or control group. For the process of randomization, first, an independent statistician generated a randomization number sequence using the SAS statistical software, version 9.2 (SAS Institute, Cary, NC, USA) with a test to control ratio of 1:1; then, the numbers were put into sequentially numbered, opaque, sealed, and stapled envelopes that contained information on the protocol of contrast media injection, which were concealed from all investigators; finally, the envelopes were delivered to a technician in charge of administering contrast media to the subject according to the instructions provided in the envelopes.
Group A (n = 52) was set as experimental group, in which, a total of 28 mL contrast agent (iopromide, Ultravist 370 mgI/mL; Bayer Healthcare, Berlin, Germany) was injected at a flow rate of 3.5 mL/s, and the iodine delivery rate (IDR) of 1.295 gI/s. Patients in the control group B (n = 52) were administered 40 mL contrast medium with the injection rate of 5 mL/s and IDR of 1.85 gI/s.
All coronary artery examinations were performed on a 3rd generation dual-source CT system (SOMATOM Force, Siemens Healthcare, Forchheim, Germany) equipped with integrated high-resolution detectors (Stellar detector, Siemens Healthcare). All patients underwent CCTA at a tube voltage of 70 kVp (Care kV set in semi mode) using prospectively electrocardiogram (ECG)-triggered high-pitch (3.2) spiral acquisition mode. Acquisition parameters were as follows: detector collimation width, 2 × 96 × 0.6 mm; gantry rotation time, 250 ms. Automated tube current modulation (CARE Dose 4D, Siemens Healthcare) was enabled and the quality reference voltage and current was set to 100 kV, 320 mAs. Image acquisition was triggered prospectively by the patient's ECG at 60% of the R-R interval. Contrast agent was injected into an antecubital vein via an 18-gauge catheter under control of the bolus tracking technique. A region of interest (ROI) was placed at the root of ascending aorta and image acquisition started 4 seconds after the attenuation signal reached a predefined threshold of 100 Hounsfield units (HU). Then, 40 mL saline solution was injected at the same injection rate after the injection of contrast medium. No premedication for HR control or vasodilatation was added to the patients' base-line medication.
All images were reconstructed using advanced modeling iterative reconstruction (ADMIRE; Siemens Healthcare) at a strength-level of 3. Slice thickness was 0.75 mm, the increment was 0.5 mm, and a medium smooth reconstruction kernel (Bv36) was selected. For patients with coronary artery stent implantation, additional images were reconstructed using a medium sharp convolution kernel (Bv49) to suppress beam hardening and blooming artefacts. All reconstructed images were transferred to a dedicated workstation (3D Workplace, Siemens Healthcare) equipped with cardiac post-processing software (Syngo.Via CT Coronary, Siemens Healthcare) for curved multiplanar reformation, maximum intensity projection, and volume rendering.
CT attenuation values in the lumen of ascending aorta root (AAO), the proximal segments of left main artery (LM), right coronary artery (RCA), left anterior descending artery (LAD) and left circumflex artery (LCX) as well as adjacent adipose tissues were measured. A circular ROI was placed in the lumen of the target vessel. The ROI was drawn as large as possible, but vessel walls, calcification, or plaques were avoided. Image noise was determined by measuring the standard deviation (SD) of CT attenuation values in the lumen ROI in the target vessel. Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were also calculated. SNR was calculated as the quotient of mean CT attenuation of the coronary artery and the corresponding image noise. CNR was determined as (CT value in coronary artery - CT value in adipose tissue) / image noise of coronary artery (19). Diagnostic IQ was considered sufficient at attenuation values > 350 HU.
The 15 coronary artery segments were determined based on the American Heart Association guidelines (20), and subjective evaluation of the CCTA IQ was conducted on coronary artery segments with a diameter of > 1.5 mm. A four-point grading scale (21) was used: score 4 (excellent IQ, clear vascular boundaries, no motion artifacts or structural discontinuity); score 3 (good IQ, mild motion artifacts); score 2 (moderate IQ, moderate motion artifacts) and score 1 (not evaluable, severe motion artifacts and poor vessel opacification). Vessel contour sharpness, and blooming artifact were also considered when evaluating the IQ of CCTA. Segments with scores of 2 to 4 were considered of diagnostic IQ; whereas, with score 1 was deemed non-diagnostic. A per-vessel and per-patient IQ score was defined as the lowest score derived from any segment for each vessel and each patient. Two senior observers (with 10 and 4 years' experience in reading CCTA, respectively) who were blinded evaluated the IQ independently and consensus was reached in a joint reading to decide the final IQ. A total of 104 patients, 415 coronary arteries, and 1348 vessel segments were included in the subjective IQ analysis.
The volumetric CT dose index (CTDI) and dose-length product (DLP) were recorded for each patient. Effective radiation dose (ED) was estimated by multiplying the DLP by a conversion factor of 0.014 mSv/(mGy x cm), as shown in the formula below:
Sample size was calculated using the PASS software version 11.0 (NCSS, LLC., Kaysville, UT, USA); group sample sizes of 47 and 47 achieved 80% power to detect non-inferiority using a one-sided, two-sample t test. The significance level (alpha) of the test was 0.05. Based on the IQ scores of our preliminary experiment, the true difference between the means was 0.070, and the margin of non-inferiority was −0.30 (10% of the means of the IQ score). The data were drawn from populations with SDs of 0.62 and 0.80. Considering a drop rate of 10%, 52 patients were finally enrolled in each group.
Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). All quantitative variables were expressed in the form of mean CT values ± SDs, whereas categorical variables were expressed in frequencies or percentages. The Kolmogorov-Smirnov test was used to test whether data was normally distributed. Independent sample t test was used to evaluate the differences in age, BMI, HR, HRV, vessel CT values, image noise, SNR, CNR, and ED between the two groups of patients. Independent sample t test was also applied to compare the IQ score rated by the two readers. Inter-observer variability between the two readers with regard to subjective IQ assessment was evaluated with κ statistics: a κ value of less than 0.20, poor agreement; a κ value of 0.21–0.40, fair agreement; a κ value of 0.41–0.60, moderate agreement; a κ value of 0.61–0.80, good agreement; and a κ value of 0.81–1.00, very good agreement. Differences in the proportions of each score between the two groups were evaluated with χ2 test. p values < 0.05 were regarded as significant.
Patients' characteristics including sex, age, mean HRs, HRV and BMI are shown in Table 1. No statistical difference in characteristics was found between the two groups, except gender distribution.
Table 2 shows the objective IQ measurements of aorta and coronary artery branches for the patients of the study population. CT values in coronary arteries for both groups were higher than 350 HU, which meet the requirements for diagnoses. Vessel CT values in group B were significantly higher than those in group A (772–851 HU vs. 436–485 HU, all p < 0.001). Image noise in group A was significantly lower than that in group B (17.8–22.3 vs. 23.3–26.4, all p < 0.005). SNRs in all vessel branches (LM excluded) in group B were higher than those in group A (32.9 ± 4.9 vs. 22.1 ± 3.7 for AAO, p < 0.001; 36.8 ± 12.3 vs. 29.2 ± 13.5 for RCA, p = 0.003; 34.4 ± 9.6 vs. 31.1 ± 13.9 for LM, p = 0.164; 37.1 ± 12.1 vs. 27.7 ± 11.3 for LAD, p = 0.001; 38.3 ± 15.5 vs. 24.3 ± 10.8 for LCX, p < 0.001). CNRs of AAO, LAD and LCX in group B were higher than those in group A (37.5 ± 5.6 vs. 27.6 ± 4.3 for AAO, p < 0.001; 42.9 ± 13.6 vs. 35.5 ± 14.3 for LAD, p = 0.009; 37.9 ± 12.0 vs. 31.2 ± 14.9 for LCX, p = 0.013). CNRs of RCA and LM showed no statistical difference in both groups (42.1 ± 13.8 vs. 36.8 ± 16.0 for RCA, p = 0.074; 38.7 ± 10.6 vs. 38.1 ± 17.0 for LM, p = 0.819).
In subjective evaluation, the IQ score exhibited no statistical difference between the two readers and interobserver agreement for IQ was very good on a per-patient basis (κ = 0.831), and good on a per-vessel and persegment basis (κ = 0.753 and 0.746, respectively).
Among the 104 patients who underwent CCTA examination, rates of diagnostic IQ were 96.1% (50/52) in group A and 94.2% (49/52) in group B, respectively, with no statistical difference (p = 0.647). Subjective scores were 3.04 ± 0.75 (group A) and 3.0 ± 0.79 (group B) (p = 0.526). In group A, 28.9% of patients (15/52) showed an image score of 4 (excellent), 55.8% (29/52) showed an image score of 3 (good), 11.5% (6/52) showed an image score of 2 (moderate) and 3.8% (2/52) showed an image score of 1 (non-diagnostic). In group B, 25.0% of patients (13/52) showed an image score of 4 (excellent), 55.8% (29/52) showed an image score of 3 (good), 13.4% (7/52) showed an image score of 2 (moderate), and 5.8% (3/52) showed an image score of 1 (non-diagnostic). No statistical difference was found in the proportion of patients with identical IQ scores (Table 3). Two patients in group A had non-diagnostic IQ due to severe coronary artery motion artifacts. Two of 3 patients in group B had non-diagnostic IQ due to severe motion artifacts, while 1 patient had non-diagnostic IQ due to severe calcification in the coronary artery.
Diagnostic IQ rates for coronary arteries were 99.1% (206/208) in group A and 98.1% (203/207) in group B, respectively, with no statistical difference (p = 0.407). Subjective image scores for coronary arteries are shown in Figure 2 and as follows: RCA, 3.34 ± 0.84 (group A) vs. 3.26 ± 0.81 (group B) (p = 0.412); LM, 3.89 ± 0.19 (group A) vs. 3.94 ± 0.31 (group B) (p = 0.690); LAD, 3.58 ± 0.52 (group A) vs. 3.67 ± 0.51 (group B) (p = 0.850); and LCX, 3.50 ± 0.49 (group A) vs. 3.33 ± 0.7 (group B) (p = 0.040). In group A, 71.2% (148/208) of coronary arteries had image scores of 4; 24.5% (51/208) of vessels, scores of 3; 3.4% (7/208) of vessels, scores of 2; and 0.9% (2/208) of 4 coronary arteries, score of 1. In group B, the proportion of coronary arteries that had image scores of 4, 3, 2, and 1 were 63.3% (131/207), 30.4% (63/207), 4.4% (9/207), and 1.9% (4/207), respectively. No statistical difference was found in the proportion of coronary arteries that showed the same IQ scores (Table 3). Four of 6 arteries were rated with non-diagnostic IQ due to severe coronary artery motion artifacts; and of these, 3 appeared in RCA and 1 in LAD. Two of 6 arteries rated with non-diagnostic image qualities that appeared in LAD and LCX, respectively, were due to calcifications in the coronary arteries.
Diagnostic IQ ratios for coronary artery segments were 99.7% (676/678) in group A and 99.4% (666/670) in group B, with no statistical difference (p = 0.405). As shown in Figure 3, the subjective scores for all coronary artery segments showed no statistical difference (p > 0.05). For the 678 segments in group A, 588 (86.7%) of the segments had IQ score of 4; 80 (11.8%) of the segments, score of 3; 8 (1.2%) of the segments, score of 2; and 2 (0.3%) of the segments, score of score 1; whereas, in group B with 670 segments, 562 (83.9%) of the segments had IQ score of 4; 93 (13.9%) of the segments, score of 3; 11 (1.6%) of the segments, score of 2; and 4 (0.6%) of the segments, score of score 1. No statistical difference was found in the proportion of coronary artery segments that had identical IQ scores (Table 3). Figures 4 and 5 show representative examples of prospectively ECG-triggered high-pitch CCTA acquired at 70 kVp with 28 mL of iodinated contrast agent.
For group A patients, the average CTDI was 1.58 ± 0.25 mGy (range, 0.81–1.99 mGy), DLP was 25.63 ± 4.43 mGycm (range, 12.5–34.9 mGycm), and ED was 0.36 ± 0.06 mSv (range, 0.18–0.49 mSv). For group B patients, CTDI was 1.59 ± 0.24 mGy (range, 0.92–2.05 mGy), DLP was 27.5 ± 5.5 mGycm (range, 13–37.2 mGycm), and ED was 0.38 ± 0.07 mSv (range, 0.22–0.54 mSv). No statistically significant differences were found for these comparisons (all p > 0.05).
This study shows that a volume of 28 mL contrast medium with 3.5 mL/s injection rate protocol was feasible to scan patients at 70 kVp with prospectively ECG-triggered high-pitch mode. For normal weight patients (BMI < 26 kg/m2) and sinus rhythm (HR < 70 bpm), CT values for all segments were > 350 HU, average radiation dose was < 0.4 mSv, the rate of diagnostic IQ for coronary arteries was 96.1%, with no statistical difference when compared with the normal contrast medium injection protocol.
Lowering the tube voltage could significantly reduce the radiation dose. Cao et al. (24) reported that 80 kVp CCTA could reduce up to 57.8% radiation dose for patients (BMI < 23 kg/m2), as compared to 120 kVp. In Zhang's study (25), the 70 kVp scanning protocol resulted in 75% reduction of the radiation dose, as compared to the 100 kVp protocol. In this study, the average radiation doses were 0.36 ± 0.06 and 0.38 ± 0.07 mSv, respectively, in the two groups, which is consistent with the results reported by Gordic et al. (26).
Although many studies have been conducted on low kV scanning, most of these studies focused on the low weight population (BMI < 23 kg/m2 or average weight of 60 kg), which is not representative of the entire population (2427). This is due to the limitation of low kV scanning in which average X-ray photon energy was reduced and a larger proportion of the radiation energy was absorbed by patients. Image noise is therefore increased, which is not conducive for diagnosis (28). Thus, higher tube current and advanced iterative image reconstruction algorithms are required to compensate for the increase in image noise. In this study, the third-generation dual-source CT was used, which has a maximum of 1300 mA tube current output for both tubes. Thus, 70 kVp scanning is more applicable to normal weight patients rather than only slim patients and children. In addition, to further reduce image noise, we used the ADMIRE algorithm, which was designed to reduce the image noise and improve the detectability of low-contrast objects while maintaining or even increasing spatial resolution and preserving the well-established image noise texture of a filtered-back projection image (17). By using ADMIRE, image noise was reduced, and both SNR and CNR were significantly improved as compared to previously reported results (25).
In addition, 70 kVp CCTA has the advantage of substantial reduction of contrast medium, because low kV imaging greatly increases the image contrast. The increase in signal intensity is explained by the X-ray absorption characteristics of iodine (15). LaBounty et al. (29) reported CT values of vessels for some patients of 700 HU when 80 kVp was used to scan patients (BMI < 25 kg/m2) using fixed amount of contrast medium and injection rate. Hell et al. (17) also used 70 kVp and 60 mL contrast media (injection rate of 5 mL/s) CCTA for obese patients (60–100 kg) and reported coronary artery CT values of 750–850 HU. This implies that there is much scope for contrast medium volume and injection rate reduction, when low kV is used for CCTA examinations. Kok et al. (30) found that 56% contrast medium dosage could be saved when 70 kVp was used for scanning as compared to 120 kVp; in addition, in their ex vivo model, 70 kVp scanning with IDR of 0.8 gI/s could achieve the CT values for conventional 120 kVp CCTA. Lell et al. (31) confirmed that with constant injection duration and adapted IDR, an identical temporal attenuation curve could be obtained for the 120 kV reference protocol with 64 mL of contrast medium and the 70 kV protocol with 32 mL of contrast medium. Based on the previous studies (143132), we used the contrast medium injection rate of 3.5 mL/s (IDR, 1.295 gI/s) and injection duration of 8 seconds; in our study, the total volume of contrast medium was reduced to 28 mL. Our results showed that this injection protocol could provide diagnostic IQ: vessel CT values were all > 350 HU and SNRs, CNRs were adequately high for diagnosis. As compared to the conventional 50–60 mL CCTA contrast medium amount (3334), a total of 50% contrast medium reduction was observed. In addition, 3.5 mL/s-injection rate enabled CCTA examinations for more patients with poor venous access.
Our study has some limitations. First, the study population was strictly filtered and, therefore, this scanning protocol is only applicable to normal-size patients. Moreover, it is not applicable to patients with high HRs, arrhythmia, or obesity. Second, only the CCTA IQ was objectively and subjectively evaluated, and the diagnostic accuracy was not compared with invasive coronary angiography.
In conclusion, prospectively ECG-triggered high-pitch CCTA at 70 kVp with 28 mL of contrast media and an injection rate of 3.5 mL/s can provide diagnostic IQ for normal-weight patients with low HRs and sinus rhythm. The proposed protocol is a suitable option to exclude cardiovascular diseases, if radiation dose or contrast media toxicity is a concern.
Figures and Tables
Fig. 2
Subjective ratings of CCTA IQ on coronary artery basis.
LCX showed significant difference in IQ between two groups (p = 0.040). y-axis represent IQ score. A = group A, B = group B, IQ = image quality, LAD = left anterior descending artery, LCX = left circumflex artery, LM = left main coronary artery, RCA = right coronary artery
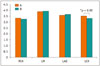
Fig. 3
Subjective ratings of CCTA IQ on coronary artery segment basis.
x-axis represents coronary artery segments. y-axis represents IQ score. A = group A, B = group B.
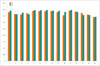
Fig. 4
65-year-old woman (HR = 56 bpm, BMI = 19.9 kg/m2, ED = 0.19 mSv) with atypical chest pain underwent CCTA.
All visualized coronary segments were evaluated as score 4 (excellent IQ).
A. CT value of AAO was 596 HU. B. Volume-rendered reformatted image of coronary tree. C-E. Curved multiplanar reformatted images of RCA, LAD, and LCX. AAO = ascending aorta root, BMI = body mass index, bpm = beats per minute, ED = effective dose, HR = heart rate, HU = Hounsfield unit
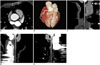
Fig. 5
CCTA in 74-year-old man (HR = 63 bpm, BMI = 24.3 kg/m2, ED = 0.38 mSv) with chest pain for one day.
A. Showed CT value of AAO was 442 HU. B. Volume-rendered. C. Curved multiplanar reformation of RCA. D. Curved multiplanar reformation showed severe stenosis in proximal segment of LAD. E. Curved multiplanar reformation of LCX showed punctuate calcification in proximal segment. IQ of CCTA study was rated as score 4 (excellent) in this patient.
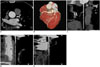
Table 1
Comparison of Patients' Characteristics in Study Population

Table 2
Comparison of Objective Measurements of CCTA Image Quality Parameters between Group A and B

Table 3
Subjective Ratings of CCTA IQ on Per-Patient, Per-Vessel, and Per-Segment Basis in Group A and B

References
1. Neefjes LA, Rossi A, Genders TS, Nieman K, Papadopoulou SL, Dharampal AS, et al. Diagnostic accuracy of 128-slice dual-source CT coronary angiography: a randomized comparison of different acquisition protocols. Eur Radiol. 2013; 23:614–622.
2. Moscariello A, Vliegenthart R, Schoepf UJ, Nance JW Jr, Zwerner PL, Meyer M, et al. Coronary CT angiography versus conventional cardiac angiography for therapeutic decision making in patients with high likelihood of coronary artery disease. Radiology. 2012; 265:385–392.
3. Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012; 366:1393–1403.
4. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ, et al. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285.
5. von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med. 2011; 154:413–420.
6. Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010; 5:4–9.
7. Morsbach F, Desbiolles L, Plass A, Leschka S, Schmidt B, Falk V, et al. Stenosis quantification in coronary CT angiography: impact of an integrated circuit detector with iterative reconstruction. Invest Radiol. 2013; 48:32–40.
8. Komatsu S, Kamata T, Imai A, Ohara T, Takewa M, Ohe R, et al. Coronary computed tomography angiography using ultra-low-dose contrast media: radiation dose and image quality. Int J Cardiovasc Imaging. 2013; 29:1335–1340.
9. Zhang C, Zhang Z, Yan Z, Xu L, Yu W, Wang R. 320-row CT coronary angiography: effect of 100-kV tube voltages on image quality, contrast volume, and radiation dose. Int J Cardiovasc Imaging. 2011; 27:1059–1068.
10. Lu C, Wang Z, Ji J, Wang H, Hu X, Chen C. Evaluation of a chest circumference-adapted protocol for low-dose 128-slice coronary CT angiography with prospective electrocardiogram triggering. Korean J Radiol. 2015; 16:13–20.
11. Achenbach S, Marwan M, Schepis T, Pflederer T, Bruder H, Allmendinger T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009; 3:117–121.
12. Lell MM, May M, Deak P, Alibek S, Kuefner M, Kuettner A, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011; 46:116–123.
13. Hou Y, Zheng J, Wang Y, Yu M, Vembar M, Guo Q. Optimizing radiation dose levels in prospectively electrocardiogram-triggered coronary computed tomography angiography using iterative reconstruction techniques: a phantom and patient study. PLoS One. 2013; 8:e56295.
14. Zhang LJ, Qi L, Wang J, Tang CX, Zhou CS, Ji XM, et al. Feasibility of prospectively ECG-triggered high-pitch coronary CT angiography with 30 mL iodinated contrast agent at 70 kVp: initial experience. Eur Radiol. 2014; 24:1537–1546.
15. Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol. 2009; 193:47–54.
16. Mihl C, Kok M, Altintas S, Kietselaer BL, Turek J, Wildberger JE, et al. Evaluation of individually body weight adapted contrast media injection in coronary CT-angiography. Eur J Radiol. 2016; 85:830–836.
17. Hell MM, Bittner D, Schuhbaeck A, Muschiol G, Brand M, Lell M, et al. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: feasibility, image quality, radiation dose, and effect of iterative reconstruction. J Cardiovasc Comput Tomogr. 2014; 8:418–425.
18. Den Harder AM, Willemink MJ, De Ruiter QM, De Jong PA, Schilham AM, Krestin GP, et al. Dose reduction with iterative reconstruction for coronary CT angiography: a systematic review and meta-analysis. Br J Radiol. 2016; 89:20150068.
19. Cademartiri F, Maffei E, Arcadi T, Catalano O, Midiri M. CT coronary angiography at an ultra-low radiation dose (< 0.1 mSv): feasible and viable in times of constraint on healthcare costs. Eur Radiol. 2013; 23:607–613.
20. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975; 51:4 Suppl. 5–40.
21. Chen MY, Shanbhag SM, Arai AE. Submillisievert median radiation dose for coronary angiography with a second-generation 320-detector row CT scanner in 107 consecutive patients. Radiology. 2013; 267:76–85.
22. Bongartz G, Golding SJ, Jurik AG, Leonardi M, van Persijn E, van Meerten R, et al. European guidelines for multislice computed tomography: appendix c funded by the European Commission. Contract number FIGM-CT2000-20078-CT-TIP.Msct.eu Web site. Accessed October 12, 2015. http://www.msct.eu/CT_Quality_Criteria.htm.
23. Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011; 5:198–224.
24. Cao JX, Wang YM, Lu JG, Zhang Y, Wang P, Yang C. Radiation and contrast agent doses reductions by using 80-kV tube voltage in coronary computed tomographic angiography: a comparative study. Eur J Radiol. 2014; 83:309–314.
25. Zhang LJ, Qi L, De Cecco CN, Zhou CS, Spearman JV, Schoepf UJ, et al. High-pitch coronary CT angiography at 70 kVp with low contrast medium volume: comparison of 80 and 100 kVp high-pitch protocols. Medicine (Baltimore). 2014; 93:e92.
26. Gordic S, Husarik DB, Desbiolles L, Leschka S, Frauenfelder T, Alkadhi H. High-pitch coronary CT angiography with third generation dual-source CT: limits of heart rate. Int J Cardiovasc Imaging. 2014; 30:1173–1179.
27. Kidoh M, Nakaura T, Nakamura S, Namimoto T, Nozaki T, Sakaino N, et al. Contrast material and radiation dose reduction strategy for triple-rule-out cardiac CT angiography: feasibility study of non-ECG-gated low kVp scan of the whole chest following coronary CT angiography. Acta Radiol. 2014; 55:1186–1196.
28. McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009; 47:27–40.
29. LaBounty TM, Leipsic J, Poulter R, Wood D, Johnson M, Srichai MB, et al. Coronary CT angiography of patients with a normal body mass index using 80 kVp versus 100 kVp: a prospective, multicenter, multivendor randomized trial. AJR Am J Roentgenol. 2011; 197:W860–W867.
30. Kok M, Mihl C, Hendriks BM, Altintas S, Kietselaer BL, Wildberger JE, et al. Optimizing contrast media application in coronary CT angiography at lower tube voltage: evaluation in a circulation phantom and sixty patients. Eur J Radiol. 2016; 85:1068–1074.
31. Lell MM, Jost G, Korporaal JG, Mahnken AH, Flohr TG, Uder M, et al. Optimizing contrast media injection protocols in state-of-the art computed tomographic angiography. Invest Radiol. 2015; 50:161–167.
32. Park EA, Lee W, Kang DK, Kim SJ, Kim YJ, Kim Y, et al. Comparison of iohexol-380 and iohexol-350 for coronary CT angiography: a multicenter, randomized, double-blind phase 3 trial. Korean J Radiol. 2016; 17:330–338.
33. Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010; 31:340–346.
34. Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008; 29:191–197.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download