Abstract
Objective
Few studies have investigated treatment strategies for brain tumor with a coexisting unruptured intracranial aneurysm (cUIA). The purpose of this study was to evaluate the safety and efficacy of preoperative coiling for cUIA, and subsequent brain tumor surgery.
Materials and Methods
A total of 19 patients (mean age, 55.2 years; M:F = 4:15) underwent preoperative coiling for 23 cUIAs and subsequent brain tumor surgery. Primary brain tumors were meningiomas (n = 7, 36.8%), pituitary adenomas (n = 7, 36.8%), gliomas (n = 3, 15.8%), vestibular schwannoma (n = 1, 5.3%), and Rathke's cleft cyst (n = 1, 5.3%). cUIAs were located at the distal internal carotid artery (n = 9, 39.1%), anterior cerebral artery (n = 8, 34.8%), middle cerebral artery (n = 4, 17.4%), basilar artery top (n = 1, 4.3%), and posterior cerebral artery, P1 segment (n = 1, 4.3%). The outcomes of preoperative coiling of cUIA and subsequent brain tumor surgery were retrospectively evaluated.
Results
Single-microcatheter technique was used in 13 cases (56.5%), balloon-assisted in 4 cases (17.4%), double-microcatheter in 4 cases (17.4%), and stent-assisted in 2 cases (8.7%). Complete cUIA occlusion was achieved in 18 cases (78.3%), while residual neck occurred in 5 cases (21.7%). The only coiling-related complication was 1 transient ischemic attack (5.3%). Neurological deterioration did not occur in any patient during the period between coiling and tumor surgery. At the latest clinical follow-up (mean, 29 months; range, 2–120 months), 15 patients (78.9%) had favorable outcomes (modified Rankin Scale, 0–2), while 4 patients (21.1%) had unfavorable outcomes due to consequences of brain tumor surgery.
Concurrent intracranial lesions can be accidentally detected by preoperative radiologic evaluation in patients with a brain tumor. Coexistent lesions are a new clinical concern to neurosurgeons. Cases of coexisting unruptured intracranial aneurysm (cUIA) are particularly worrying to neurosurgeons since cUIA rupture can be fatal during the perioperative period (12). Previous studies reported that the incidence of cUIA associated with brain tumor ranged from 2.3% to 7.7% (3456789). Considering the variable incidence of cUIA associated with brain tumors (3456789), there are a considerable number of cases in literature documenting perioperative aneurysm rupture (1011121314). This data emphasizes the importance of cUIA management before tumor treatment (121011121314). However, few studies have investigated treatment strategies for brain tumor with cUIA (1516171819). Preoperative coiling and subsequent tumor surgery is one strategic option for treating cUIA prior to brain tumor surgery. However, few cases exist in literature, which were treated by this strategy (171819).
The purpose of our study is to evaluate the safety and effectiveness of preoperative coiling for cUIA and subsequent brain tumor surgery, in patients with brain tumor and cUIA.
This retrospective study was approved by the institutional review board and informed patient consent was waived for study inclusion based on study characteristics. Radiologic and clinical data were recorded in a prospectively maintained neurointerventional database, and were retrospectively reviewed.
Between November 2003 and November 2014, the patients who underwent preoperative coiling for cUIA and subsequent brain tumor surgery were identified from a prospectively maintained neurointerventional database of a single tertiary referral academic hospital. All patients were clinically assessed at the time of admission and at post-treatment (coiling and brain tumor surgery). Clinical outcomes were evaluated at the latest clinical follow-up on an outpatient basis or by telephone interview. All cUIAs were identified during preoperative evaluation of the known brain tumor. Each case was discussed by the neurointerventionists and neurosurgeons for determining the treatment strategy. Coiling prior to tumor surgery was considered based on at least one of the following conditions: 1) aneurysm with maximal size ≥ 5 mm, which is an internal criteria of the study hospital for preoperative coiling, 2) neighboring aneurysm near the tumor, which potentially compromise tumor surgery, 3) expected unstable hemodynamic situation during the perioperative period (highly vascular tumor or sinus invasion by tumor), or 4) demand by the oncologic neurosurgeon, based on the operative plan. Informed consent for treatment was obtained from the patient or legal representatives.
All coiling procedures were performed under general anesthesia (n = 18) or intravenous sedation (n = 1). After femoral access, a 6-Fr Envoy (Cordis, Miami Lakes, FL, USA) guiding catheter or shuttle guiding sheath (Cook, Bloomington, IN, USA) was introduced into the internal carotid or vertebral artery. A bolus of 3000 IU heparin was administered intravenously, and maintained with a dose of 1000 IU/hour. If stent-assist coiling was planned, dual anti-platelet agents were prescribed to the patient for at least 5 days before the procedure. Dual anti-platelet medication was maintained for 3 months post-operation, and then changed to aspirin monotherapy for patients with stent-assisted coiling. Coiling technique and angiographic outcomes were retrospectively evaluated. Angiographic outcome was determined by Raymond's classification.
Procedure-related complications, defined as any clinical deterioration (either transient or permanent) after treatment, were evaluated by immediate postprocedural imaging studies, electronic medical records, and a neurointerventional database. Clinical outcomes were determined at the latest clinical follow-up on an outpatient basis, or by telephone interview according to the modified Rankin Scale (mRS). Favorable outcomes were defined as mRS 0–2.
Nineteen patients (15 female) underwent preoperative coiling for 23 cUIA and subsequent brain tumor surgery. The mean age was 55.2 years, ranging from 33 to 73 years.
Primary tumors were meningiomas (n = 7, 36.8%), pituitary adenomas (n = 7, 36.8%), gliomas (n = 3, 15.8%), vestibular schwannoma (n = 1, 5.3%), and Rathke's cleft cyst (n = 1, 5.3%). cUIAs were located at the distal internal carotid artery (n = 9, 39.1%), anterior cerebral artery (n = 8, 34.8%), middle cerebral artery (n = 4, 17.4%), basilar artery top (n = 1, 4.3%), and posterior cerebral artery P1 (n = 1, 4.3%). The characteristics of these patients are summarized in Table 1.
The single-microcatheter technique was used in 13 cases (56.5%), balloon-assisted was used in 4 cases (17.4%), double-microcatheter was used in 4 cases (17.4%), and stent-assisted was used in 2 cases (8.7%). Complete cUIA occlusion was achieved in 18 cases (78.3%), while residual neck occurred in 5 cases (21.7%). No technical failures were noted. Follow-up MR or catheter angiogram was performed in 15 patients (78.9%) with 18 cUIA at 6 months or more (mean 25 months). There were 14 cases (77.8%) of "stable", 2 cases (11.1%) of "minor recurrence", and 2 cases (11.1%) of "major recurrence", respectively. One of the "major recurrence" was successfully re-treated by additional simple coiling.
Clinical outcomes after preoperative cUIA coiling and tumor surgery are summarized in the Table 1. Transient ischemic attack (grade 4 contralateral hemiparesis) occurred after coiling in 1 patient. This was the only case of coiling-related complications (5.3%). At mean follow-up of 29 months, 15 patients (78.9%) had favorable outcomes (mRS, 0–2). There was 1 case (5.3%) of coiling-related complication and 4 cases (21.1%) of tumor surgery-related complications.
The median interval between coiling and tumor surgery was 55 days (range, 2–211 days). Neurological deterioration was not observed in any patient during the period between coiling and tumor surgery. Tumor surgery-related complications occurred in 4 patients (21.1%): hydrocephalus (n = 1), hemorrhage (n = 1), subdural hematoma infection (n = 1), and motor weakness due to brain edema after removal of a large meningioma (n = 1). In these patients, aneurysms were treated by simple coiling (n = 3) or balloon-assisted coiling (n = 1). No anti-platelet agents were administered after coiling. Clinical follow-up was achieved in 19 patients, with a mean follow-up period of 29 months after the final treatment (range, 2–120 months). Favorable outcomes were observed in 15 patients (78.9%). Among them, initial functional status of 2 patients (#17 and #18) was mRS 2 due to hemiparesis by malignant brain tumor, and did not changed after coiling and tumor surgery. Meanwhile, 4 patients (21.1%) had unfavorable outcomes at the latest follow-up. Causes of unfavorable outcomes were ischemic stroke after radiation therapy (n = 1), recurrence and multiple metastasis of underlying renal cell carcinoma (n = 1), weakness after brain tumor surgery (n = 1), and sepsis (n = 1) from subdural hematoma infection (Table 1).
The overall outcomes of patients after treatment of both cUIAs and tumors were favorable in 78.9% of patients and unfavorable in 21.1% of patients during a mean of 29 months (range, 2–120 months) follow-up. No patient had any neurological deterioration during the period between coiling and tumor surgery. All unfavorable outcomes were related to brain tumor-related complications, tumor progression, or underlying malignancy (renal cell carcinoma).
With the recent development of neuroimaging techniques, cUIAs have been increasingly detected along with primary brain tumors. In this study, since all brain tumor patients were not evaluated with vascular imaging (MR/CT or catheter angiogram) due to its retrospective nature, the overall incidence of cUIA with brain tumor could not be analyzed. Previous studies reported that the incidence of cUIA associated with brain tumor ranges from 2.3% to 7.0%, and the most common combination was a meningioma (3456789).
Treatment strategies for brain tumor and cUIA have not yet been established. Because the tumor is responsible for presenting symptoms in most cases, treatment strategies should focus on tumor surgery (16). If a cUIA is small and distantly located from the brain tumor, tumor surgery can be performed first and the cUIA can be followed up or treated later. However, depending on the cUIA location and size, the cUIA should be treated prior to tumor surgery. For cUIAs adjacent to brain tumors (Figs. 1, 2), the aneurysm should be treated prior to tumor surgery because the aneurysm may be inadvertently injured and rupture during or after tumor surgery (101112131415). The risk of rupturing a cUIA, especially a large one (Fig. 3), may increase due to hemodynamic changes during or after tumor removal. We had one case of aneurysm rupture shortly after tumor surgery (unpublished data).
Several strategic options exist when an untreated cUIA poses a threat during or after tumor surgery. Simultaneous aneurysm clipping and tumor removal is occasionally possible, depending on the anatomical relationship between the tumor and cUIA (15). In general, this strategy is technically more demanding, and may increase the possibility of complications during or after tumor surgery. Another option is a staged operation to clip the cUIA with subsequent tumor surgery. However, this involves repeat craniotomy and delays tumor surgery, more than the preoperative coiling strategy. Endovascular treatment of UIA is very safe and effective even in wide neck and large aneurysms (20212223). Thus, cUIA coiling and subsequent tumor surgery is an alternative option. However, only a few cases treated by this strategy are reported in literature (171819). To our knowledge, this study is the first case series collected over an 11-year period at a single large center, where the safety and efficacy of preoperative coiling and subsequent brain tumor surgery has been evaluated.
This strategy has several advantages. First, it eliminates the concern of possible severe complications associated with cUIA during tumor surgery. It is a more simplified tumor surgery than the one stage operation of cUIA and brain tumor. Second, this technique alleviates the burden of repeat craniotomy. Finally, delay time for tumor surgery is lesser than the strategy of clipping and subsequent tumor surgery. One major concern about preoperative coiling is the use of antiplatelet medication with stents. In our study, only 2 aneurysms (8.7%) were treated with stent-assisted coiling due to wide neck (dome to neck ratio ≤ 1.5) and under-tall (aspect ratio ≤ 1.0) aneurysm. These patients had slowly growing benign tumors without urgent clinical symptoms, and a delay in tumor surgery did not matter. Another major concern is that periprocedural complications of coiling might delay tumor operations. In general, however, the morbidity of coiling for cUIA is very low. In our series, there was only 1 transient ischemic attack, which neither delayed tumor surgery nor affected the clinical outcome.
This study has several limitations. First, due to its retrospective nature, interval between coiling and tumor surgery was very variable (2–211 days), and indication for preoperative coiling of cUIA was not strict. In 2 patients with stent-assisted coiling, antiplatelet medication delayed tumor surgery. Meanwhile, in the other patients, surgery was delayed due to a slow growing benign tumor, preoperative medical treatment, and elective operation schedule. Secondly, a small number of cases in this study may limit the generalizability of study results. However, prevalence of cUIA with brain tumor is very low, the effectiveness and safety of preoperative coiling for cUIA were focused in this study.
In cases of cUIA and brain tumor, treatment strategies should be designed according to the tumor and aneurysm conditions, locations, and pathologic nature. Preoperative cUIA coiling generally has low morbidity, can simplify tumor surgery, and results in fewer tumor surgery delays than the strategy of staged clipping with subsequent tumor surgery. If cUIA treatment is needed prior to tumor surgery, preoperative cUIA coiling with subsequent tumor surgery is a good treatment option.
In conclusion, Preoperative coiling with subsequent tumor surgery was safe and effective. It is a reasonable treatment option for patients with concurrent brain tumor and cUIA.
Figures and Tables
Fig. 1
53-year-old woman presenting with pituitary adenoma and coexisting unruptured aneurysm.
A. Coronal view of T2 weighted MRI shows 5.3-mm sized aneurysm (arrow) buried in PA. Arrowheads indicate right internal carotid artery. B. Oblique coronal view of flat panel angiographic CT shows small aneurysm (arrow) arising from right internal carotid artery, with dome buried in PA. C. Control angiogram after coiling shows complete aneurysm occlusion. D. 6-month follow-up MR angiogram shows complete occlusion state of aneurysm (white arrow). PA = pituitary adenoma

Fig. 2
55-year-old man presenting with high grade glioma and concurrent unruptured aneurysm.
A. Axial view of T2 weighted MRI shows 7.7-mm sized aneurysm (arrow) at anterior communicating artery and brain tumor with cystic portion (T) in left frontotemporal region. Note dome of aneurysm is toward brain tumor. B. Working projection view of angiogram during coiling. C. Control angiogram after coiling shows complete occlusion of aneurysm. D. Axial view of T2 weighted MRI after tumor surgery. Arrow indicated coiled aneurysm.
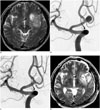
Fig. 3
68-year-old woman presenting with left parietal convexity meningioma and coexisting unruptured aneurysm.
A. Sagittal view of enhanced MR shows meningioma with peritumoral edema in left parietal convexity. B. Lateral projection of left internal carotid angiogram shows large aneurysm with daughter sac at left posterior communicating artery origin. C. Control angiogram after coiling shows complete aneurysm occlusion. D. MR-DWI obtained due to transient ischemic attack (grade 4 contralateral hemiparesis) shows several high signal spots. MR-DWI = magnetic resonance diffusion weighted imaging
E. Axial view of T2 weighted MRI after tumor removal. Patient had grade 4 contralateral weakness after brain tumor surgery, but recovered over several weeks. F. 2-year follow-up MR angiogram shows complete occlusion state of aneurysm (white arrow). 2-year Functional status of this patient was mRS 1. mRS = modified Rankin Scale
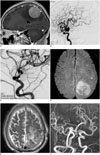
Table 1
Results of Preoperative Coiling and Subsequent Tumor Surgery in Patients with Brain Tumors and Concurrent Unruptured Aneurysms

AChA = anterior choroidal artery, Acom = anterior communicating artery, An. = aneurysm, BA = basilar artery, Cx = complication, F = female, ICA = internal carotid artery, Ix = indication, Loc = location, M = male, Major = recuurence requiring additional treatment, MCA = middle cerebral artery, Minor = recurrence but not requiring additional treatment, mRS = modified Rankin scale score, PCA = posterior cerebral artery, Pcom = posterior communicating artery, Pt-No = Patient number, RCC = renal cell carcinoma, SDH = subdural hematoma, Stable = improved or no interval change
References
1. Tsuchida T, Tanaka R, Yokoyama M, Sato H. Rupture of anterior communicating artery aneurysm during transsphenoidal surgery for pituitary adenoma. Surg Neurol. 1983; 20:67–70.
2. Taylor PE. Delayed postoperative hemorrhage from intracranial aneurysm after craniotomy for tumor. Neurology. 1961; 11:225–231.
3. Javalkar V, Guthikonda B, Vannemreddy P, Nanda A. Association of meningioma and intracranial aneurysm: report of five cases and review of literature. Neurol India. 2009; 57:772–776.
4. Oh MC, Kim EH, Kim SH. Coexistence of intracranial aneurysm in 800 patients with surgically confirmed pituitary adenoma. J Neurosurg. 2012; 116:942–947.
5. Oshino S, Nishino A, Suzuki T, Arita H, Tateishi A, Matsumoto K, et al. Prevalence of cerebral aneurysm in patients with acromegaly. Pituitary. 2013; 16:195–201.
6. Pant B, Arita K, Kurisu K, Tominaga A, Eguchi K, Uozumi T. Incidence of intracranial aneurysm associated with pituitary adenoma. Neurosurg Rev. 1997; 20:13–17.
7. Fischer BR, Palkovic S, Holling M, Niederstadt T, Jeibmann A, Wassmann H. Coexistence of cerebral aneurysm and meningioma--pure accident? Clin Neurol Neurosurg. 2009; 111:647–654.
8. Jakubowski J, Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978; 41:972–979.
9. Kim YH, Lee YJ, Han JH, Ahn S, Lee J, Kim JH, et al. Association of intracranial aneurysms and meningiomas: a case-control study. J Neurosurg. 2015; 123:357–361.
10. Akutsu N, Hosoda K, Ohta K, Tanaka H, Taniguchi M, Kohmura E. Subarachnoid hemorrhage due to rupture of an intracavernous carotid artery aneurysm coexisting with a prolactinoma under cabergoline treatment. J Neurol Surg Rep. 2014; 75:e73–e76.
11. Cheng WY, Shen CC. Minimally invasive approaches to treat simultaneous occurrence of glioblastoma multiforme and intracranial aneurysm -- case report. Minim Invasive Neurosurg. 2004; 47:181–185.
12. Hoya K, Yoshimoto Y, Shin M, Nemoto S. Rupture of an internal carotid artery aneurysm within a clinoidal meningioma following stereotactic radiosurgery. Acta Neurochir (Wien). 2011; 153:1995–1996.
13. Rustagi T, Uy EM, Rai M, Kannan S, Senatus P. Intracranial hemorrhage from undetected aneurysmal rupture complicating transphenoidal pituitary adenoma resection. Conn Med. 2011; 75:393–398.
14. Berker M, Aghayev K, Saatci I, Palaoğlu S, Onerci M. Overview of vascular complications of pituitary surgery with special emphasis on unexpected abnormality. Pituitary. 2010; 13:160–167.
15. Zhong Z, Sun Y, Lin D, Sun Q, Bian L. Surgical treatment of brain tumor coexisted with intracranial aneurysm--case series and review of the literature. Neurosurg Rev. 2013; 36:645–656. discussion 656.
16. Licata C, Pasqualin A, Freschini A, Barone G, Da Pian R. Management of associated primary cerebral neoplasms and vascular malformations: 1. intracranial aneurysms. Acta Neurochir (Wien). 1986; 82:28–38.
17. Yamada S, Yamada SM, Hirohata T, Ishii Y, Hoya K, Murakami M, et al. Endoscopic extracapsular removal of pituitary adenoma: the importance of pretreatment of an adjacent unruptured internal carotid artery aneurysm. Case Rep Neurol Med. 2012; 2012:891847.
18. Xia X, Ramanathan M, Orr BA, Salmasi V, Salvatori R, Reh DD, et al. Expanded endonasal endoscopic approach for resection of a growth hormone-secreting pituitary macroadenoma coexistent with a cavernous carotid artery aneurysm. J Clin Neurosci. 2012; 19:1437–1441.
19. Yu K, Herwadkar A, Kearney T, Gnanalingham KK. Pituitary adenoma and incidental superior hypophyseal aneurysm. Br J Neurosurg. 2011; 25:432–433.
20. Cho YD, Rhim JK, Kang HS, Park JJ, Jeon JP, Kim JE, et al. Use of triple microcatheters for endovascular treatment of wide-necked intracranial aneurysms: a single center experience. Korean J Radiol. 2015; 16:1109–1118.
21. Cho YD, Rhim JK, Park JJ, Jeon JS, Yoo RE, Kang HS, et al. Microcatheter looping to facilitate aneurysm selection in coil embolization of paraclinoid aneurysms. Korean J Radiol. 2015; 16:899–905.
22. Kim BM, Shin YS, Baik MW, Lee DH, Jeon P, Baik SK, et al. Pipeline embolization device for large/giant or fusiform aneurysms: an initial multi-center experience in Korea. Neurointervention. 2016; 11:10–17.
23. Kwon WH, Jeong HW, Kim ST, Seo JH. Angiographic and clinical result of endovascular treatment in paraclinoid aneurysms. Neurointervention. 2014; 9:83–88.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download