Abstract
Objective
To determine whether triple arterial phase acquisition via a combination of Contrast Enhanced Time Robust Angiography, keyhole, temporal viewsharing and parallel imaging can improve arterial phase acquisition with higher spatial resolution than single arterial phase gadoxetic-acid enhanced magnetic resonance imaging (MRI).
Materials and Methods
Informed consent was waived for this retrospective study by our Institutional Review Board. In 752 consecutive patients who underwent gadoxetic acid-enhanced liver MRI, either single (n = 587) or triple (n = 165) arterial phases was obtained in a single breath-hold under MR fluoroscopy guidance. Arterial phase timing was assessed, and the degree of motion was rated on a four-point scale. The percentage of patients achieving the late arterial phase without significant motion was compared between the two methods using the χ2 test.
Results
The late arterial phase was captured at least once in 96.4% (159/165) of the triple arterial phase group and in 84.2% (494/587) of the single arterial phase group (p < 0.001). Significant motion artifacts (score ≤ 2) were observed in 13.3% (22/165), 1.2% (2/165), 4.8% (8/165) on 1st, 2nd, and 3rd scans of triple arterial phase acquisitions and 6.0% (35/587) of single phase acquisitions. Thus, the late arterial phase without significant motion artifacts was captured in 96.4% (159/165) of the triple arterial phase group and in 79.9% (469/587) of the single arterial phase group (p < 0.001).
Liver magnetic resonance imaging (MRI) with hepatobiliary contrast agents such as gadoxetic acid or gadobenate dimeglumine has been increasingly used for the evaluation of focal liver lesions in recent years, as it provides strong lesion-to-liver contrast via hepatocyte-specific uptake during the hepatobiliary phase (12). In particular, there is ample evidence demonstrating the diagnostic value of gadoxetic acid-enhanced MRI for the detection of small hepatocellular carcinomas (HCCs) (3), and for the differentiation between HCCs and other hepatic lesions or hypervascular pseudolesions (2). Thus, at present, several recent guidelines include gadoxetic acid-enhanced MRI as a primary diagnostic imaging modality for HCC along with extracellular contrast media-enhanced dynamic contrast-enhanced CT and MRI (4567). However, obtaining the arterial phase at sufficient quality is more challenging in gadoxetic acid-enhanced liver MRI, due to the narrow time window of the arterial phase resulting from the smaller amount of the standard dose (8), as well as a high incidence of transient dyspnea in comparison with extracellular contrast agents (910). Therefore, in order to improve the gadoxetic acid-enhanced MRI quality of the arterial phase, several strategies have been suggested including slowing down the injection rate to prolong the arterial phase (1112), the use of double-dose gadoxetic acid (13), and reduction of the scan acquisition time (914). However, reduction of the injection rate may lead to unintended reduction of the peak enhancement degree; in addition, administration of double-dose gadoxetic acid reportedly increases the incidence of transient dyspnea (15) ruining the image quality of the arterial phase. Furthermore, reduction of the scan acquisition time often has a trade-off with decreased spatial resolution and signal-to-noise ratio (SNR), which may lead to less diagnostic images (916).
Recently, several techniques of parallel imaging and viewsharing (1617181920) or non-Cartesian acquisition (202122) have been applied to contrast-enhanced dynamic MRI, so as to achieve high spatio-temporal resolution, which would potentially improve the overall arterial image quality at gadoxetic-enhanced MRI. Although the use of twodimensional (2D) parallel imaging techniques facilitates acquisition of spatial resolution three-dimensional (3D) T1-gradient-echo (GRE) sequences at reduced acquisition time while controlling unwrapping artifacts, reduced SNR is inevitable due to reduced number of acquired echoes compared with conventional 3D T1 GRE sequence (2324). Although non-Cartesian acquisition schemes are robust to motion artifacts, their imaging characteristics are rather different from standard 3D GRE techniques with Cartesian acquisition and require longer reconstruction times and higher computational hardware demands (2122). Until now, there have been a few studies dealing with multiple arterial phase acquisition using the Cartesian acquisition scheme that did not need to compromise spatial resolution (1625). Hadizadeh et al. (18) recently reported that viewsharing in keyhole imaging and the 2D parallel imaging technique with high reduction factors could allow for increased spatial and temporal resolution in time-resolved MR angiography in the brain. However, to our knowledge, there has been no study regarding the clinical benefit of the high spatial resolution multi-arterial phase using this combination of techniques performed in a large population with liver diseases in comparison with single arterial phase acquisition in combination with MR fluoroscopy. Thus, the purpose of this study was to determine whether triple arterial phase (TAP) imaging can improve arterial phase imaging with high spatial resolution in comparison to single arterial phase acquisition on gadoxetic-acid enhanced MRI.
This retrospective study was approved by our Institutional Review Board and the requirement for informed consent was waived.
From October 2012 to January 2013, a total of 752 consecutive patients (M:F = 504:248; mean age, 60.4 years for men and 59.7 years for women) (Table 1) were referred to our MR units for routine contrast-enhanced liver MRI according to radiology database search. All patients underwent gadoxetic-acid enhanced liver MR using three different scanners of our institution as scheduled. Among them, 21.9% (165/752) underwent liver MRI with TAP acquisition at a 3T scanner (Ingenia, Philips Healthcare, Best, the Netherlands), and the others underwent liver MRI with a single arterial phase at either 3T (33.0%, 248/752; Magnetom Verio, Siemens Healthcare, Erlangen, Germany) or 1.5T (45.1%, 339/752; Signa HDx, GE Healthcare, Wakesha, IL, USA) scanners depending on the scanners' availability for time-resolved T1-weighted 3D GRE sequences, which can be used for acquiring multiphasic arterial phase imaging during the study period. Between the two groups, there were no significant differences in demographics except for the high incidence of chronic liver disease in the single arterial acquisition group (p = 0.03) and higher incidence of oncology patients in the TAP acquisition group (p = 0.005) (Table 1).
The routine liver MR sequence consisted of precontrast heavily T2-weighted images, diffusion weighted images, precontrast T1-weighted images (T1WI) and dynamic contrast-enhanced T1WI, regardless of the scanner type. After intravenous injection of a standard dose of contrast media (0.025 mmol/kg, Primovist or Eovist, Bayer Healthcare, Berlin, Germany) at 1.5 mL/sec followed by 25 mL saline chaser, arterial, portal venous, transitional and hepatobiliary phases were obtained. In all scanners, arterial phase timing was determined under MR fluoroscopy guidance (26) as each vendor recommended. In scanner 1, 2D fluoroscopy slice was positioned in descending aorta and monitored every second, and arterial phase is acquired when the descending aorta enhances. In scanner 2, right atrium is monitored every second after contrast media injection, and arterial phase is scanned eight seconds later right atrium shows enhancement. In scanner 3, descending aorta signal intensity reaches to a predefined level using dynamic care bolus, arterial phase is scanned. Detailed scanning parameters of the arterial phase in each scanner were listed in Table 2. In one scanner (Ingenia, Philips Healthcare, Best, the Netherlands), three arterial phases were obtained in 165 patients in one breath-hold, and the three arterial phases were referred as 1st, 2nd, and 3rd TAP scans according to the scan order of multiarterial phase as below. The applied techniques for acquisition of TAPs in one breath hold were originally described in a previous study of time resolved MR angiography for cerebral vessels (18). The applied technique consisted of contrast enhanced time robust angiography (CENTRA) (17, 27), keyhole, viewsharing (18) and the 2D parallel imaging technique with reduction factors of two in phase, and two in slice-encoding directions (28). In brief, a central K-space (Ky-Kz) is sampled repeatedly, and an elliptical-centric readout of the periphery of K-space is obtained as the last step of the acquisition (29) serving as the reference scan. To improve time resolution, elliptical central K-space is additionally divided into three keyhole fractions including central (C) and two peripheries (P+, P-) and the fractions are acquired in alternative repetition of C and P+ pair followed by C and P- pair (Fig. 1). The alternative acquisition between CP+ and CP-, and the pseudorandom order sampling in each fraction contribute to ensure that there is no discontinuity of signal intensity between K-space areas. The proportion of elliptical central K-space, which can be modified by users, was 20% in this study, and the viewsharing percentage (center of central K-space/the whole central K-space) was 60%. Alternation mode was not applied in our study, so as not to reduce data fidelity. The breath-hold time for the three arterial phases was 18 to 21 seconds, i.e., 6 to 7 seconds per phase.
Two attending radiologists reviewed the images independently, and discrepant cases were reviewed additionally in consensus a week from the independent review session. All image reviews were performed using picture archiving and communication system workstations (Maroview 5.4, Infinitt, Seoul, Korea) with monitors having a spatial resolution of 1600 × 1200 (Totoku, Tokyo, Japan). The timing of the arterial phase was graded on a three point-scale (916): 1 = early arterial phase, only hepatic artery is opacified; 2 = late arterial phase, opacified hepatic artery and mild portal vein enhancement, without strong parenchymal enhancement or hepatic vein enhancement; 3 = portal venous phase, opacification of the portal vein, and opacification of the hepatic vein.
Respiratory motion artifacts were scored on a four point-scale (916): 1 = non-diagnostic due to severe motion; 2 = motion-related artifacts causing impaired diagnostic capability of the readers; 3 = noticeable motion-related artifacts with an image quality decrease, but no diagnostic performance impairment; and 4 = no or minimal artifacts. Typical feature of truncation artifact presented as ringing artifact along the liver margin is not considered as motion artifact (3031). Otherwise, artifacts were considered as combination of motion artifact and truncation artifact, and scored accordingly. In patients who underwent liver MR including TAPs, image quality was also evaluated in addition to timing and respiratory motion in every phase. The overall image quality was graded on a four-point scale (14): score 1, diagnostically unacceptable; score 2, worse than average; score 3, average; and score 4, good and better than average. For comparison between single arterial phase and TAPs, the single phase of the best timing adequacy (score 2) was chosen among the three arterial phases, and the motion score of the determined best arterial phase was the representative motion score. If there were more than two arterial phases with the same timing adequacy, the highest motion score was selected.
Finally, one attending radiologist reviewed the arterial and transitional phases of all patients and classified them into 'no motion', 'transitional' or 'persistent' motion categories depending on the presence of motion artifacts on the arterial phase as well as on the transitional phase(9). Patients who showed motion artifacts on at least one arterial phase was regarded as patients with 'transient' or 'persistent' motion depending on the presence of motion on the transitional phase.
The χ2 test was performed to compare categorical variables and the Student t test or Mann-Whitney test was used for continuous variable comparison between single and TAPs, as appropriate. Comparison of motion artifacts, timing adequacy, and image quality was done among the scanners and three arterial phases using the Kruskal-Wallis test or Friedman analysis with pairwise comparison using Bonferroni correction. Agreement between the two reviewers for every item was evaluated using weighted kappa stastitics. A κ value of 0 indicated poor; 0.01–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, excellent agreement (32). All statistical analyses were performed using commercially available software (MedCalc, version 12, MedCalc Software, Mariakerke, Belgium; IBM SPSS Statistics, version 22.0, SPSS Inc., Armonk, NY, USA). A p value < 0.05 (p < 0.017 in three comparisons after Bonferroni correction) was considered to indicate a statistically significant difference.
In patients with TAP acquisition, the late arterial phase was achieved at least once in 96.4% (159/165) of patients; the late arterial phase was missed in 6 patients in whom only the early arterial phase (n = 1) and portal venous phase (n = 5) were obtained through TAPs. Capture of the late arterial phase was observed in 83% (137/165), 84.2% (139/165), and 73.3% (121/165) of the patients in the 1st, 2nd, and 3rd scans, respectively. In patients with conventional single arterial phase acquisition, the late arterial phase was observed in 84.2% (494/587, 81.4% [276/339] at 1.5T; and 87.9% [218/248] at 3T). In 12.6% (74/587) patients, the early arterial phase was obtained and the portal venous phase was captured in 3.2% of (19/587) patients (Table 3). In short, TAP acquisition showed a significantly higher chance of obtaining the late arterial phase compared to conventional, single arterial phase acquisition (96.4% [159/165] vs. 84.2% [494/587], p < 0.001) (Fig. 2).
Late arterial phase imaging without noticeable motion artifacts (motion score of 4) was obtained at least once in 86.1% (142/165) patients, and the late arterial phase without significant motion artifacts (motion score ≥ 3) was achieved at least once in 96.4% (159/165) of patients. In patients with single arterial phase, 60.0% (352/587) showed late arterial phase with motion score 4 and 19.9% (117/587) showed late arterial phase with motion score 3. In 156 patients who underwent TAP, 3.6% (6/165) patients showed missed late arterial phase or significant motion artifact (score ≤ 2) on all three scans, whereas the incidence was 20.1% (118/587) in single arterial phase acquisition. The rate of obtaining the late arterial phase without significant motion artifacts was significantly higher in TAP acquisition than in conventional single acquisition (96.4% [159/165] vs. 79.9% [469/587], p < 0.001). In detail, among the 165 patients with TAP acquisition, the 1st scan showed higher motion artifact than the others (3.4 ± 0.8 on 1st, 3.8 ± 0.4 on 2nd, 3.8 ± 0.4 on 3rd, p < 0.001) and showed lower image quality compared with the 2nd and 3rd scans (3.0 ± 0.8, 3.7 ± 0.6, 3.7 ± 0.6, respectively, p < 0.001). However, the 2nd and 3rd scans did not show a significant difference of motion artifacts and image quality (p = 0.34, 0.20, respectively) (Table 3).
In the 165 patients who underwent TAP acquisition, noticeable motion artifacts (score ≤ 3) were significantly reduced on the 2nd and 3rd scans (13.9% [23/165] and 13.9% [23/165], respectively), compared with the 1st scan (40% [66/165]). In addition, compared with patients who underwent only single arterial phase (31.9% [187/587]), there was no significant difference between the single arterial phase and the 1st arterial scan of triple acquisition (p = 0.06). However, the 2nd and 3rd scans of triple acquisition showed less motion artifacts (3.6 ± 0.5, 3.5 ± 0.7, respectively) than the single arterial phase (2.8 ± 0.5, p < 0.001, p < 0.001, respectively). Significant motion artifacts (score ≤ 2) were observed in 13.3% (22/165), 1.2% (2/165), 4.8% (8/165) on 1st, 2nd, and 3rd scans of TAP acquisitions and 6.0% (35/587) of single phase acquisitions (p = 0.003, 0.02, 0.70, respectively). A detailed summary of the motion score and image quality was summarized in Table 3.
Among the 253 patients with noticeable motion, 235 patients (62 in the TAP group and 173 in the single arterial phase group) were categorized as the 'transient motion group'. Among them, 56 patients showed significant motion during arterial phase (22 in the TAP group and 34 in the single arterial phase group). In 62 patients who underwent TAP acquisition, motion was most frequently observed in the 1st scan (48.4%, 30/62). Significant motion was limited on the 1st scan in 63.6% (14/22) of patients. The most frequently affected arterial phase timing was the late arterial phase (72.6%, 45/62) followed by the early arterial phase and early + late arterial phase (9.7%, 6/62), after matching the scan order with the captured arterial phase timing (Table 4, Fig. 3). In one patient who showed motion artifacts (score 3) on only the portal venous phase, all three arterial scans were obtained on the portal venous phase due to missed timing (Table 4). In patients who underwent single arterial phase acquistion, the late arterial phase was the most frequently affected phase (75.7% [131/173]) (Table 4).
Reader agreement for the arterial phase and motion artifacts were good to excellent in arterial phase timing (0.80 in the 1st TAP scan, 0.92 in the 2nd TAP scan, 0.90 in the 3rd TAP scan, 0.91 in the conventional single phase), motion artifacts (0.78 in the 1st TAP scan, 0.90 in the 2nd TAP scan, 0.83 in the 3rd TAP scan, 0.86 in the conventional single phase), and image quality (0.85 in the 1st TAP scan, 0.73 in the 2nd TAP scan, 0.81 in the 3rd TAP scan).
Our study results revealed that a combination of CENTRA, keyhole, viewsharing and the 2D parallel imaging technique contributed to the reliable attainment of the "late arterial phase" at gadoxetic acid-enhanced liver MRI, which is critical for the detection of hypervascular liver tumors, even though previously regarded as challenging (914). In our study, the proportion of capturing the late arterial phase was 96.4% (159/165) in patients with TAP acquisition, which was significantly higher than the 84.2% (494/587) in patients who underwent conventional single arterial phase acquisition (p < 0.001). In fact, in previous studies, bolus-tracking methods such as the MR fluoroscopy-guided technique or the test bolus technique showed a higher rate of obtaining the optimal arterial phase than fixed delay scans (333435). However, missed arterial phases were reported in more than 10–15% of patients even after using the bolus-tracking technique due to either operator dependent problem or patient-related variables such as cardiac output (36). In our study, the combination of single breath-hold TAP acquisition and MR fluoroscopy guided technique provided the optimal arterial phase in most patients on at least one of the three phases, and the rate of capturing the late arterial phase was higher than the success rate of TAP acquisition with a fixed delay (9), and in fact, comparable with five-arterial phase acquisition with a fixed-delay (16). Thus, our results may provide clinically valuable information on optimal arterial phase acquisition at gadoxetic acid-enhanced liver MRI.
In our study, high temporal resolution as well as high spatial resolution was achieved, i.e., a combination of alternating viewsharing to keyhole techniques reduced scan times while maintaining high spatial resolution. Furthermore, the pseudorandom sampling of each partition of central K-space contributed to ensuring that there were no signal discontinuities throughout the keyhole acquisition (18) or ringing artifacts (27). Previously, in order to solve the problem of transient severe motion in the arterial phase of gadoxetic acid-enhanced MR imaging, a similar approach of multiple arterial phase imaging was applied, but high spatial resolution was undervalued as high temporal resolution with relatively low spatial resolution was reported to be clinically feasible in a prior study (9). However, gadoxetic acid-enhanced liver MRI can serve as a problem-solving tool for challenging cases in clinical practice and preoperative imaging for liver malignancies (37). Given that high spatial resolution contributes to better image quality and conspicuity of lesions and anatomic structures for preoperative planning (283839), achieving high temporal and spatial resolution MRI would be of clinical importance.
Another obstacle to achieving the optimal late arterial phase at liver MRI may be motion artifacts. Owing to the relatively long acquisition time of MRI compared with computed tomography, motion artifacts are often encountered in patients with limited breath-holding capacity (40). Furthermore, transient dyspnea or transient severe motion after contrast media administration has been more frequently reported in patients who undergo gadoxetic acid-enhanced liver MRI than extracellular agents (101540). The incidence of motion-related artifact has varied in the literature (10294041), but one recent study reported that 39% of cases showed motion-related artifacts on the arterial phase at gadoxetic acid-enhanced MRI (10). Although transient dyspnea is a self-limited benign phenomenon, it should be avoided by protocol adjustment if possible as it frequently deteriorates the arterial phase image quality, which thereby would hamper the diagnosis, leading to repetition of the examination. Several risk factors including the presence of chronic liver disease and a history of transient dyspnea have been suggested (91041), but a recent study reported that transient dyspnea is poorly predicted by any of the reported risk factors (42). Thus, such results may require implementation of the arterial phase sequence, which would be clinically feasible and applicable to most patients, not only for the high-risk group.
In our study, significant motion artifacts were observed in 7.6% (57/752) of patients during the arterial phase. In 35 patients who underwent conventional single arterial phase acquisition, not only image quality but also diagnostic performance was negatively affected on qualitative evaluation. However, in the remaining 22 patients who underwent TAP acquisition, only one patient had persistent significant motion artifacts, which affected the diagnostic performance of radiologists. Thus, TAP acquisition would provide appropriate arterial phase acquisition with appropriate arterial timing and reduced motion contamination.
Of note, we found that motion artifacts were observed on the 1st phase of the three arterial scans in all patients who had transient motion artifacts, i.e., none of the patients showed motion artifact limited to 2nd and 3rd TAP scans. Indeed, 48.4% (30/62) of patients with noticeable motion artifacts and 63.6% (14/22) of patients with significant motion artifacts were in the 1st TAP scan. Although we had a limitation in the determination of the timing of motion on the basis of reconstructed images due to viewsharing and sharing the reference scan throughout the reconstruction, it is likely that motion may occur only in the early period of the arterial phase when motion artifacts are restricted on the 1st TAP scan. Therefore, although we could not capture the onset timing of motion, our observation suggests that nearly half of the transient motion occurred in the early phase after contrast media injection, and would typically cease in a short period. Our study results seem to be discrepant with the literature reporting that the 3rd scan of consecutive three arterial phases has seen a significant proportion of motion artifacts compared with the 1st and 2nd TAP scans (9). We do not have sufficient data to explain the discrepancy between these two studies. However, we postulate that different acquisition methods might have contributed to our differing results; we used MR fluoroscopy guided arterial phase acquisition whilst 15 to 20 seconds a fixed-delay method was used in the literature (9). In addition, another possible cause might be that prolonged arterial phase acquisition time up to 23 seconds by obtaining three phases in the previous study (9) could have contributed to initiating or worsening motion artifacts on the last phase due to the patients' shortage of breath-holding capacity.
Our study has several limitations. First, its retrospective nature may have had an inevitable bias. Second, the acquisition time of the TAP imaging (18–21 seconds) was not the same compared with that of single arterial phase imaging (17–19 seconds). Third, transient dyspnea occurring before starting arterial phase acquisition might have led to underestimation of its incidence. In addition, motion artifacts were assessed by image analysis indirectly which may have also led to underestimation of the incidence of transient motion. However, investigation of the incidence of transient dyspnea was not our primary goal; instead, we focused on the feasibility of multi-arterial phases in clinically problematic cases, not asymptomatic and clinically acceptable liver MRI. Fourth, contamination of truncation artifact might be a source of error to estimate motion artifact although we tried to minimize contamination. Fifth, one may argue that the techniques used in our study may not be as promising as newly introduced techniques such as compressed sensing. However, our study purpose is not comparing the superiority of a technique to another but investigating whether the techniques would contribute to achieving optimal arterial phase consistently. Finally, we did not evaluate additional values for detecting lesions on three arterial images. However, we showed an improved rate of obtaining reliable arterial phase imaging, which would be clinically valuable, contributing to the achievement of a thorough, full data set of gadoxetic acid-enhanced liver MRI.
In conclusion, TAP imaging may reliably provide adequate arterial phase imaging for gadoxetic acid-enhanced liver MRI.
References
1. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–50. PMID: 25247563.

2. Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, et al. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (< or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010; 45:96–103. PMID: 20057319.
3. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. PMID: 25559230.

4. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y. Liver Cancer Study Group of Japan. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014; 87(Suppl 1):7–21. PMID: 25427729.

5. Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015; 61:1056–1065. PMID: 25041904.

6. Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, et al. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015; 40:613–625. PMID: 25287679.

7. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522. PMID: 25995680.
8. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009; 44:305–310. PMID: 19462484.

9. Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014; 271:426–434. PMID: 24475864.

10. Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014; 272:123–131. PMID: 24617733.

11. Tamada T, Ito K, Yoshida K, Kanki A, Higaki A, Tanimoto D, et al. Comparison of three different injection methods for arterial phase of Gd-EOB-DTPA enhanced MR imaging of the liver. Eur J Radiol. 2011; 80:e284–e288. PMID: 21296514.

12. Kim SM, Heo SH, Kim JW, Lim HS, Shin SS, Jeong YY, et al. Hepatic arterial phase on gadoxetic acid-enhanced liver MR imaging: a randomized comparison of 0.5 mL/s and 1 mL/s injection rates. Korean J Radiol. 2014; 15:605–612. PMID: 25246821.

13. Motosugi U, Ichikawa T, Sano K, Sou H, Onohara K, Muhi A, et al. Double-dose gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic liver disease. Invest Radiol. 2011; 46:141–145. PMID: 21139506.

14. Park YS, Lee CH, Kim IS, Kiefer B, Woo ST, Kim KA, et al. Usefulness of controlled aliasing in parallel imaging results in higher acceleration in gadoxetic acid-enhanced liver magnetic resonance imaging to clarify the hepatic arterial phase. Invest Radiol. 2014; 49:183–188. PMID: 24276676.

15. Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK. Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol. 2014; 203:796–802. PMID: 25055154.

16. Hope TA, Saranathan M, Petkovska I, Hargreaves BA, Herfkens RJ, Vasanawala SS. Improvement of gadoxetate arterial phase capture with a high spatio-temporal resolution multiphase three-dimensional SPGR-Dixon sequence. J Magn Reson Imaging. 2013; 38:938–945. PMID: 23371926.

17. Beck GM, De Becker J, Jones AC, von Falkenhausen M, Willinek WA, Gieseke J. Contrast-enhanced timing robust acquisition order with a preparation of the longitudinal signal component (CENTRA plus) for 3D contrast-enhanced abdominal imaging. J Magn Reson Imaging. 2008; 27:1461–1467. PMID: 18504734.

18. Hadizadeh DR, Gieseke J, Beck G, Geerts L, Kukuk GM, Boström A, et al. View-sharing in keyhole imaging: partially compressed central k-space acquisition in time-resolved MRA at 3.0 T. Eur J Radiol. 2011; 80:400–406. PMID: 20447790.
19. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential Subsampling with Cartesian Ordering (DISCO): a high spatio-temporal resolution Dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012; 35:1484–1492. PMID: 22334505.

20. Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, et al. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver:advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014; 24:1290–1299. PMID: 24633374.
21. Agrawal MD, Spincemaille P, Mennitt KW, Xu B, Wang Y, Dutruel SP, et al. Improved hepatic arterial phase MRI with 3-second temporal resolution. J Magn Reson Imaging. 2013; 37:1129–1136. PMID: 23197440.

22. Kim KW, Lee JM, Jeon YS, Kang SE, Baek JH, Han JK, et al. Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with K-space weighted image contrast (KWIC). Eur Radiol. 2013; 23:1352–1360. PMID: 23187728.

23. Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK, Choi BI. High-resolution T1-weighted gradient echo imaging for liver MRI using parallel imaging at high-acceleration factors. Abdom Imaging. 2014; 39:711–721. PMID: 24557640.

24. Yu MH, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Clinical application of controlled aliasing in parallel imaging results in a higher acceleration (CAIPIRINHA)-volumetric interpolated breathhold (VIBE) sequence for gadoxetic acid-enhanced liver MR imaging. J Magn Reson Imaging. 2013; 38:1020–1026. PMID: 23559147.

25. Budjan J, Ong M, Riffel P, Morelli JN, Michaely HJ, Schoenberg SO, et al. CAIPIRINHA-Dixon-TWIST (CDT)-volumeinterpolated breath-hold examination (VIBE) for dynamic liver imaging: comparison of gadoterate meglumine, gadobutrol and gadoxetic acid. Eur J Radiol. 2014; 83:2007–2012. PMID: 25172427.

26. Merkle EM, Dale BM. Abdominal MRI at 3.0 T: the basics revisited. AJR Am J Roentgenol. 2006; 186:1524–1532. PMID: 16714640.

27. Willinek WA, Gieseke J, Conrad R, Strunk H, Hoogeveen R, von Falkenhausen M, et al. Randomly segmented central k-space ordering in high-spatial-resolution contrast-enhanced MR angiography of the supraaortic arteries: initial experience. Radiology. 2002; 225:583–588. PMID: 12409598.

28. Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK, Choi BI. Fat-suppressed, three-dimensional T1-weighted imaging using high-acceleration parallel acquisition and a dual-echo Dixon technique for gadoxetic acid-enhanced liver MRI at 3 T. Acta Radiol. 2015; 56:1454–1462. PMID: 25480475.
29. Hoogeveen R, von Falkenhausen M, Gieseke J. Fast dynamic, high resolution contrast-enhanced MR angiography with CENTRA keyhole and SENSE [abstract]. Proc Int Soc Mag Reson Med. 2004; 12:9.
30. Tanimoto A, Higuchi N, Ueno A. Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci. 2012; 11:91–97. PMID: 22790295.

31. Huh J, Kim SY, Yeh BM, Lee SS, Kim KW, Wu EH, et al. Troubleshooting arterial-phase MR images of gadoxetate disodium-enhanced liver. Korean J Radiol. 2015; 16:1207–1215. PMID: 26576109.

32. Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977; 33:363–374. PMID: 884196.

33. Haradome H, Grazioli L, Tsunoo M, Tinti R, Frittoli B, Gambarini S, et al. Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging. 2010; 32:334–340. PMID: 20677259.

34. Sharma P, Kalb B, Kitajima HD, Salman KN, Burrow B, Ray GL, et al. Optimization of single injection liver arterial phase gadolinium enhanced MRI using bolus track real-time imaging. J Magn Reson Imaging. 2011; 33:110–118. PMID: 21182128.

35. Nakamura S, Nakaura T, Kidoh M, Utsunomiya D, Doi Y, Harada K, et al. Timing of the hepatic arterial phase at Gd-EOBDTPA-enhanced hepatic dynamic MRI: comparison of the testinjection and the fixed-time delay method. J Magn Reson Imaging. 2013; 38:548–554. PMID: 23744782.

36. Hussain HK, Londy FJ, Francis IR, Nghiem HV, Weadock WJ, Gebremariam A, et al. Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradientrecalled-echo sequence: comparison with test-bolus method. Radiology. 2003; 226:558–566. PMID: 12563155.

37. Cho JY, Lee YJ, Han HS, Yoon YS, Kim J, Choi Y, et al. Role of gadoxetic acid-enhanced magnetic resonance imaging in the preoperative evaluation of small hepatic lesions in patients with colorectal cancer. World J Surg. 2015; 39:1161–1166. PMID: 25609116.

38. Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK, Choi BI. Fatsuppressed, three-dimensional T1-weighted imaging using high-acceleration parallel acquisition and a dual-echo Dixon technique for gadoxetic acid-enhanced liver MRI at 3 T. Acta Radiol. 2015; 56:1454–1462. PMID: 25480475.
39. AlObaidy M, Ramalho M, Busireddy KK, Liu B, Burke LM, Altun E, et al. High-resolution 3D-GRE imaging of the abdomen using controlled aliasing acceleration technique - a feasibility study. Eur Radiol. 2015; 25:3596–3605. PMID: 25916391.

40. Wile GE, Leyendecker JR. Magnetic resonance imaging of the liver: sequence optimization and artifacts. Magn Reson Imaging Clin N Am. 2010; 18:525–547. PMID: 21094454.

41. Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013; 266:452–461. PMID: 23192781.

42. Kim SY, Park SH, Wu EH, Wang ZJ, Hope TA, Chang WC, et al. Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol. 2015; 204:1220–1227. PMID: 26001231.

Fig. 1
K-space acquisition scheme using combination of CENTRA, keyhole and viewsharing.
K-space is divided into central (Kz-Ky) and peripheral portion (R), and central K-space is sampled repeatedly in random manner whilst peripheral K-space is acquired once last to serve as reference scan for each reconstruction (violet arrows). Central K-space is divided into three keyhole fractions including central (C) and two peripheries (P+, P-) and fractions are acquired in alternating fashion between combinations of (P+, C) and (C, P-). To avoid signal discontinuation, P+ or P- of keyhole obtained on previous scan is added to reconstruction of next scan (orange arrows). CENTRA = contrast enhanced time robust angiography
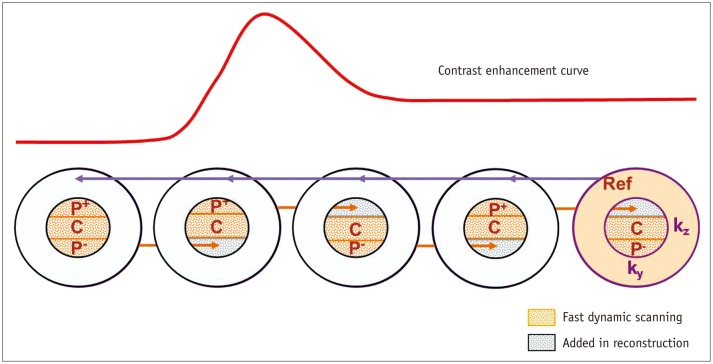
Fig. 2
Triple arterial phase (TAP) of 80-year-old man with colon cancer.
1st, 2nd, and 3rd TAP scans (A-C) provide early arterial phase (A) and two late arterial phases (B, C) with different parenchymal enhancement degrees.
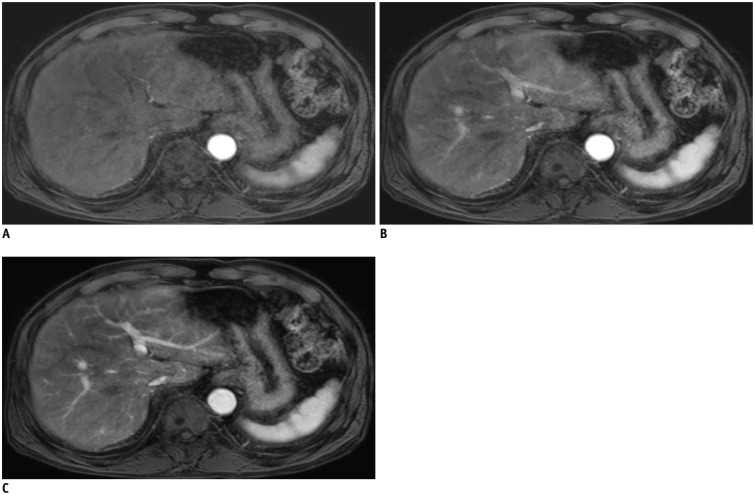
Fig. 3
Fig. 3. Triple arterial phase of 70-year-old woman with colon cancer liver metastasis.
1st scan was deteriorated by significant motion artifacts with truncation artifact, leading to non-diagnostic image (A). Following 2nd (B) and 3rd (C) TAP scans were regarded as optimal late arterial phase imaging, and successfully demonstrate 4 cm rim enhancing mass which was confirmed as metastasis after surgery (arrows). TAP = triple arterial phase
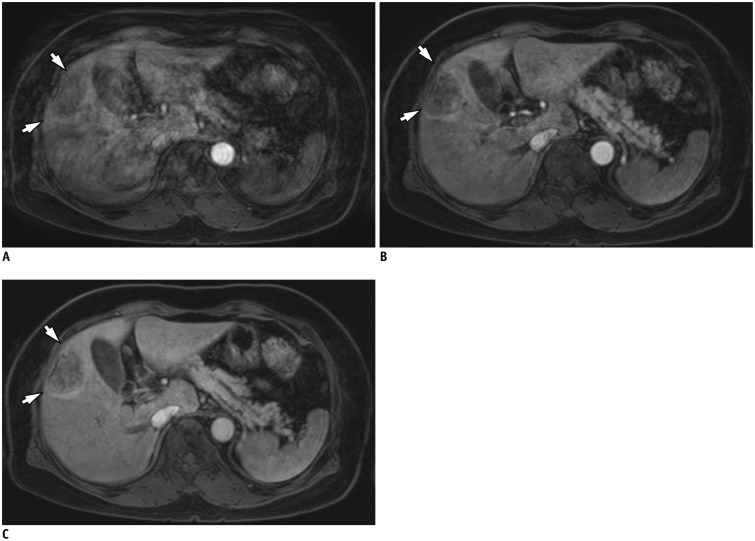
Table 1
Patient Characteristics
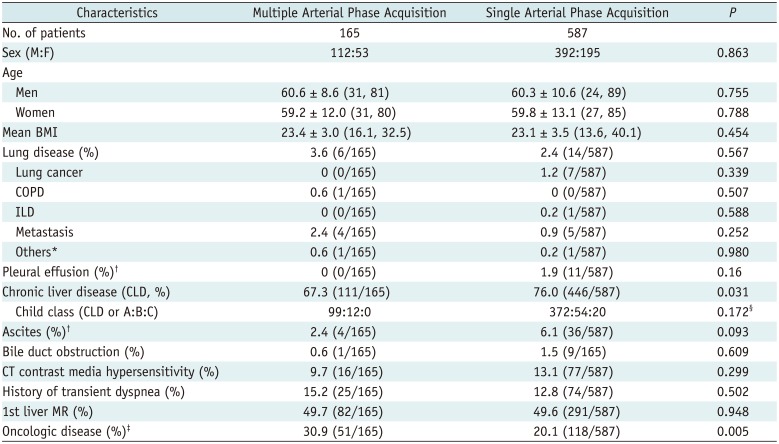
Values are presented as mean ± standard deviation (range). *One pulmonary ateriovenous malformation and one tuberculosis, †Moderate to large amount, ‡Any oncologic disease except hepatocellular carcinoma, §Comparison between two groups regarding incidence of child B or C to child A. BMI = body mass index, COPD = chronic obstructive lung disease, ILD = interstitial lung disease
Table 2
Scan Parameters of Fat Suppressed 3D T1-Weighted APs in Each MR Scanner
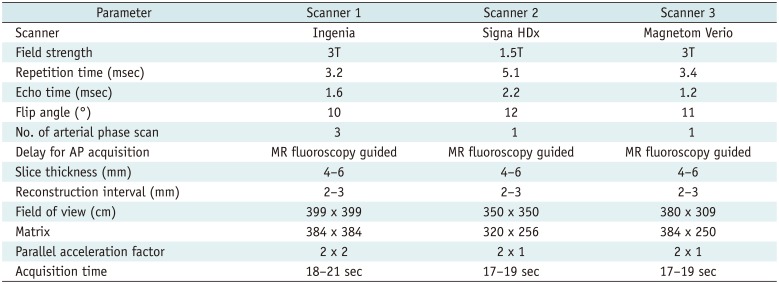
Table 3
Captured Arterial Phase, Motion Artifacts, and Image Quality in Triple Arterial Phase (TAP) and Conventional Single Arterial Phase Acquisition
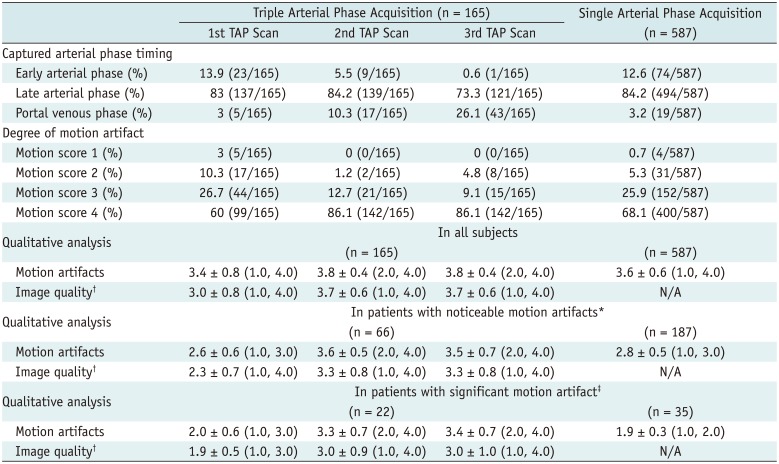
Table 4
Frequency of Motion Artifacts at Each Scan and Captured Arterial Phase Timing in 225 Patients with Only Transient Motion Artifacts




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download