Abstract
Objective
To compare computed tomography (CT) and magnetic resonance imaging (MRI) findings between two histological types of nasal hemangiomas (cavernous hemangioma and capillary or lobular capillary hemangioma).
Materials and Methods
CT (n = 20; six pre-contrast; 20 post-enhancement) and MRI (n = 7) images from 23 patients (16 men and seven women; mean age, 43 years; range, 13-73 years) with a pathologically diagnosed nasal cavity hemangioma (17 capillary and lobular capillary hemangiomas and six cavernous hemangiomas) were reviewed, focusing on lesion location, size, origin, contour, enhancement pattern, attenuation or signal intensity (SI), and bony changes.
Results
The 17 capillary and lobular hemangiomas averaged 13 mm (range, 4-37 mm) in size, and most (n = 13) were round. Fourteen capillary hemangiomas had marked or moderate early phase enhancement on CT, which dissipated during the delayed phase. Four capillary hemangiomas on MRI showed marked enhancement. Bony changes were usually not seen on CT or MRI (seen on five cases, 29.4%). Half of the lesions (2/4) had low SI on T1-weighted MRI images and heterogeneously high SI with signal voids on T2-weighted images. The six cavernous hemangiomas were larger than the capillary type (mean, 20.5 mm; range, 10-39 mm) and most had lobulating contours (n = 4), with characteristic enhancement patterns (three centripetal and three multifocal nodular), bony remodeling (n = 4, 66.7%), and mild to moderate heterogeneous enhancement during the early and delayed phases.
Although hemangiomas are common lesions of the head and neck, those of the nasal cavity are rare (1). Hemangiomas are classified as either cavernous or capillary types; capillary hemangiomas also include lobular capillary hemangiomas (1, 2). The predominant type of nasal hemangioma is a capillary hemangioma, which usually arises from the nasal septum, whereas cavernous hemangiomas are more likely to be found on the lateral wall of the nasal cavity (3).
Until now, the radiological features of nasal hemangiomas have been almost exclusively reported as case reports or small case series (1, 4, 5), and the names associated with hemangiomas, such as capillary hemangioma, lobular capillary hemangioma, cavernous hemangioma, organizing hematoma, and angiomatous polyp, are very confusing. Although there is only one large series describing the imaging findings of lobular capillary hemangiomas compared with those of inverted papillomas (6), no study has evaluated the radiological features of sinonasal hemangiomas according to histological type. In our study, we evaluated computed tomography (CT) and magnetic resonance imaging (MRI) findings of 23 patients with confirmed nasal cavity hemangiomas, according to two histological types, such as capillary hemangiomas (including lobular capillary types) and cavernous hemangiomas.
This retrospective study was approved by the Institutional Review Board, and informed consent was waived.
We reviewed CT (n = 20) and MRI (n = 7) findings of 23 patients with histologically diagnosed nasal cavity hemangiomas. The patients were recruited based on a computer search of a pathological database from January 1998 to December 2012. All patients received curative surgical excision of their tumors at our institution. We did not re-evaluate any pathological specimens for the current study. At the pathological analysis, the hemangiomas were categorized into the capillary and cavernous subtypes according to the dominant composing vessel size under a microscope.
There were 16 men and seven women (age, 13-73 years; mean age, 43.5 years): 17 patients (12 men and five women; age, 3-67 years; mean age, 42.5 years) were diagnosed with capillary hemangiomas (including lobular capillary hemangiomas), and six patients (four men and two women; age, 16-73 years; mean age, 46.3 years) were diagnosed with cavernous hemangiomas.
Fourteen patients presented with epistaxis, 11 with nasal obstruction, and two with both epistaxis and nasal obstruction. The mean interval between presentation of clinical symptoms and the physical examinations was 12.7 months (range, 2 days-10 years) for all patients, and the intervals were 9.1 and 22.8 months for capillary hemangiomas and cavernous hemangiomas, respectively.
Tumor location and origin (categorized as the nasal septum or lateral wall including the turbinates), surgical findings, and imaging were evaluated.
Among the 23 patients, 20 received CT, seven received MRI, and four received both procedures. All CT and MRI examinations included contrast-enhanced imaging. All CT scans were dual-phase enhanced images of both early and delayed phases. Six CT examinations also contained pre-contrast images.
CT examinations were typically performed using a helical CT scanner (Genesis Highspeed; GE Medical Systems, Milwaukee, WI, USA) or a multidetector CT (MDCT) scanner (Somatom Sensation 16; Siemens Medical Systems, Erlangen, Germany). Early-phase axial 3-mm images were obtained 45 seconds after intravenously administering 60-120 mL of non-ionic contrast medium. Delayed-phase coronal images (by helical CT scanner) or delayed axial and reconstructed coronal images (by MDCT scanner) were obtained 120 seconds after contrast injection.
MRI was performed using a 1.5-T MR Scanner (Signa Genesis or Signa Excite; GE Medical Systems) with a head coil. Pre-contrast T1-weighted spin echo images (repetition time [TR]/echo time [TE]/number of excitation [NEX], 450-560 ms/10-14 ms/2), T2-weighted fast spin echo images (TR/TE/NEX, 2200-4600 ms/80-110 ms/1), with or without fat saturation, and contrast-enhanced T1-weighted images, with or without fat saturation, were obtained from all patients after injecting 0.1 mmol/kg gadopentetate dimeglumine. Images were obtained in at least two planes with a 3-4 mm slice thickness, 0-0.4 mm interslice gap, 256 × 192 matrix, and a 22 cm field-of-view.
All CT and MRI images were respectively reviewed by two head and neck radiologists with 14 and 4 years of experience in head and neck imaging by consensus. We evaluated the CT and MRI images with an emphasis on lesion location, size, contour, enhancement pattern, attenuation or signal intensity (SI), signal void, and associated bony changes. We described the tumor location as the anterior nasal cavity (when the tumor was in the nasal cavity anterior to the turbinates), middle meatus (tumor was between the middle and inferior turbinate), inferior meatus (tumor was inferior to the inferior turbinate), maxillary sinus, and ethmoid sinus. Then, we compared these imaging characteristics between the histological subtypes.
Lesion size was calculated based on mean maximum diameter measured in three orthogonal planes. Contour was categorized as round to ovoid or lobulating. Lesion attenuation on pre-contrast CT was compared to attenuation of the masticator muscles by visual assessment. Lesion SI was compared to that of the spinal cord gray matter or cerebrum included in the scan. The enhancement pattern of the solid areas on the post-contrast CT and MRI images was categorized as homogeneous or heterogeneous. The degree of lesion enhancement on CT was defined by visual comparison to masticator muscles and extracranial vessel attenuation. A tumor was considered mildly enhanced when it demonstrated similar attenuation to the muscle on the delayed phase; markedly enhanced, when similar to early-phase arterial attenuation; and moderately enhanced when the enhancement degree was in between. Bony structural changes were described as expansion, pressure remodeling, and bone erosion or destruction.
The patient's demographic features, initial symptoms, and clinical findings are summarized in Table 1.
Mean diameter of the capillary hemangiomas (including lobular capillary hemangiomas) (n = 17) was 13 mm (range, 4-37 mm). The margins were well defined in all cases (100%), and the surface contours were round to ovoid in 14 cases (82.4%) and lobulating in three (17.6%). The lesions were located primarily in the anterior nasal cavity (n = 13, 76.5%); the remainder were located near the middle meatus (n = 5, 29.4%), inferior meatus (n = 1, 5.9%), and maxillary sinus (n = 1, 5.9%). Three lesions were located in the anterior nasal cavity to the middle meatus, the middle meatus to the maxillary sinus, and the middle meatus to the inferior meatus. Nine lesions arose from the nasal septum (52.9%), and seven arose from the lateral wall (41.1%). We were unable to determine the origin on one case due to missing data. In contrast, mean diameter of the cavernous hemangiomas (n = 6) was 20.5 mm (range, 10-39 mm), and the majority (n = 4, 67%) had a lobulating contour. In three cases, tumors were located only in the middle meatus (50%); one in the anterior nasal cavity, one in the anterior nasal cavity extending into the maxillary and ethmoid sinus, and the remaining one was in both the maxillary sinus and the middle meatus. Two cavernous hemangiomas arose from the nasal septum; two others arose from the lateral wall. In the remaining two cases, the origin was not exactly determined in the surgical field, but they possibly originated from either the lateral wall or the maxillary sinus, considering the mass location.
The CT and MR imaging findings are summarized in Table 2.
The capillary hemangiomas were iso-attenuating on the four available pre-contrast CT images. Capillary hemangiomas (n = 14) showed predominantly marked enhancement on
early-phase CT images in 13 cases (92%) (Fig. 1A), which were homogeneous in eight and heterogeneous in five. The remaining tumor showed moderate heterogeneous enhancement on early-phase images. Thirteen (92%) of the tumors showed mild enhancement on delayed-phase images, whereas one showed moderate enhancement, which was homogeneous in 10 and heterogeneous in four. The prominent strong enhancement on early-phase images dissipated on the delayed-phase images (Fig. 1B) in all cases. The cavernous hemangiomas were iso-attenuating or iso- to high attenuating on the two available pre-contrast CT images. All cavernous hemangiomas showed partial heterogeneous enhancement on CT with moderate (n = 3), mild to moderate (n = 2), and mild (n = 1) enhancement during the early phase (Fig. 2A). More than half of the cavernous hemangiomas (n = 4) showed heterogeneously mild or mild to moderate enhancement on delayed-phase images, and one showed homogeneous mild enhancement (Fig. 2B). Delayed-phase images were not available in one case due to an artifact. The enhancement patterns of cavernous hemangiomas were centripetal (n = 3) or multifocal nodular (n = 3).
CT imaging revealed a peritumoral cystic area in two of the capillary hemangioma cases (2/14, 14.2%).
Two tumors of four capillary hemangiomas (Fig. 3) evaluated by MRI had low SI, one had iso-SI, and the other had low to iso-SI on T1-weighted images. Three tumors had high SI on T2-weighted images, and one had high and low SI. Three lesions showed heterogeneous SI, and one showed homogeneous SI on both T1- and T2-weighted images. All four tumors showed marked enhancement on enhanced MRI. Three cases had central or peripheral heterogeneous enhancement, whereas one had homogeneously strong enhancement throughout the tumor. Both T1- and T2-weighted images showed heterogeneously low to high SI in the nasal masses on the available MRI images (n = 3) of the cavernous hemangioma cases (Fig. 2). Two cavernous hemangiomas showed central partial enhancement on MRI and homogeneous enhancement throughout the tumor in the other case. Two had marked enhancement (67%), and one (33%) had moderate enhancement on MRI.
Signal voids on T2-weighted images were noted in the three capillary hemangiomas (75%) (Fig. 3B) and in one of the cavernous hemangiomas (33%). One of the capillary hemangiomas contained a peritumoral cystic lesion, which had low SI on T1-weighted images without enhancement (Fig. 3C).
The majority of capillary hemangiomas (70.5%) (Fig. 1) did not show bony remodeling or erosion. Adjacent bony changes were noted in five capillary hemangiomas (29.4%), and four had a mean diameter > 2 cm and were a contralateral deviation of the nasal septum and a smooth, nonaggressive bone erosion of the adjacent nasal lateral wall, inferior turbinate, or middle turbinate. In contrast, CT and MRI revealed bone structural change in four of six cavernous hemangiomas (66.7%), and all were > 2 cm in mean diameter (Fig. 2B). Three cases showed benign-appearing smooth bony remodeling, such as nasal septal deviation, widening of the ethmoid sinus and infundibulum, and uncinated process or middle turbinate remodeling. Three cases showed pressure erosion of the nasal turbinates and septum and the uncinated process. Bony changes in the cavernous hemangiomas included remodeling or erosion with smooth, nonaggressive cortical breakthrough. Bone destruction, which is a malignant tumor characteristic, was not observed in any case.
Hemangiomas are the most common tumors of the head and neck in children (7). They are histologically divided into capillary and cavernous types, depending on the dominant microscopic vessel size (8). Capillary hemangiomas are composed of capillary sized vessels lined with flattened epithelial cells separated by a collagen stroma. Lobular capillary hemangiomas, previously known as pyogenic granulomas, are a benign polypoid form of capillary hemangioma that primarily occurs in the skin and mucous membranes. They are histologically characterized by submucosal vascular proliferation and are arranged in lobules or clusters comprising central capillaries and smaller ramifying tributaries (9, 10). Cavernous hemangiomas are composed of large endothelium-lined vascular spaces (2).
Unlike other regions of the head and neck, hemangiomas in the sinonasal cavity are very rare (1, 3, 11). In a review by Ash and Old (11) of 3000 nasal polyp cases, only 23 cases of true sinonasal hemangiomas were found.
Capillary hemangiomas are considered a congenital true vascular tumor that usually tends to involute during childhood, and only a minority persists through adulthood (1). In contrast, lobular capillary hemangiomas are acquired true vascular tumors that are occasionally related to hormonal changes or trauma (5, 12, 13, 14, 15). However, it may be difficult to distinguish one from the other on a histological examination because their histological features are quite similar, in that both lesions are composed of lobules of capillaries surrounding fibrous tissue (5, 16). In the present study, we could not re-evaluate pathological slides because a large proportion of the specimens were unavailable due to the long study period. As our capillary hemangiomas were found mostly in adults, we considered capillary and lobular capillary hemangiomas as one group.
In a previous report, three of six capillary hemangiomas were accompanied by adjacent bone remodeling (1). However, most capillary hemangiomas in our series, which were < 2 cm, showed neither benign remodeling nor bone destruction. Only five lesions, measuring 1.6-3.7 cm in diameter, showed benign-looking bone remodeling and erosion on CT and MRI. According to previous studies, cavernous hemangiomas can cause extensive erosion of adjacent bone (4, 17, 18). We observed four cases (66.7%) of cavernous hemangiomas, which were > 2 cm in diameter, demonstrated erosion of the nasal turbinate or medial wall of the maxillary sinus. The bony changes were seen mostly in capillary and cavernous hemangiomas > 2 cm. We speculate that bony changes may depend on tumor size and location rather than on the histological type of hemangioma.
CT findings of sinonasal hemangiomas have been reported previously in small series (1, 4, 5, 15). Capillary hemangiomas are typically described as well-circumscribed masses with no internal calcification and homogeneous enhancement (1). In our series, most capillary and lobular capillary hemangiomas were well circumscribed, ovoid to round shaped, and had marked enhancement at the early phase, which dissipated at the delayed phase on dual-phase CT images. Yang et al. (6) described the dynamic MRI enhancement pattern of lobular capillary hemangiomas, and most (75%) of the tumors showed a washout pattern on time intensity curves. This dynamic MRI enhancement pattern corresponds well to the CT enhancement pattern we found in our study. The CT imaging findings of lobular capillary hemangiomas in previous studies were soft tissue density masses with post-obstructive secretion in Lance et al. (15) or intensely enhancing masses with an iso- or hypo-attenuating cap during contrast-enhanced CT (5). In our study, two relatively larger-sized (> 2 cm) capillary hemangiomas on CT and one on MRI had similar findings to those described by Lee et al. However, relatively larger capillary hemangiomas (including the lobular capillary type) showed more heterogeneous enhancement patterns than those of the smaller ones. Although Lee et al. (5) proposed the peripheral cap as a characteristic finding of nasal cavity lobular capillary hemangiomas, possibly due to superficial ulceration, we believe that it may have been due to combined nasal polyps.
The characteristic CT findings of cavernous hemangiomas are large, inhomogeneous masses (4). All of our cases had a heterogeneous enhancement pattern of either a centripetal or multifocal nodular pattern. The cavernous hemangiomas were larger than the capillary and lobular capillary hemangiomas. The dramatic enhancement difference between the early and delayed phases was not noted in cavernous hemangiomas compared to that in capillary and lobular capillary hemangiomas.
In Dillon's study, capillary hemangiomas had intense enhancement on MRI, and 50% (2/4) of cases had a peripheral hypo-intense rim surrounding a central mixed SI mass on T2-weighted images, which was later identified as a thrombus on a pathological examination (1). In our study, three (75%) of the capillary and lobular hemangiomas, except one case, were low or iso- to low signal masses on T1-weighted images and central masses surrounding a peripheral hypo-intense rim on T2-weighted images, which was similar to Dillon's study. In our series, contrast-enhanced T1-weighted images showed marked enhancement in capillary hemangiomas. The MRI appearance of these lesions was similar to that described by Dillon et al. (1). In our series, three cases (two capillary hemangiomas and one lobular capillary hemangioma) had signal voids within the masses. Vascular flow voids encountered on MRI in our study and previous studies are consistent with the hypervascular nature of capillary hemangiomas (1, 2, 17). Previous studies describing the MRI findings of sinonasal cavernous hemangiomas are limited (17, 18). In a case report by Dufour et al. (17), a homogeneous iso-intense nonenhancing lesion was observed on a T1-weighted image with mild hyperintense SIs on T2-weighted images. In contrast, a case report described by Vargas and Castillo (18) revealed a centrally enhancing mass with heterogeneously high and low SIs on T1- and T2-weighted images in the maxillary sinus and nasal cavity. The MRI characteristics observed in our cavernous hemangiomas were mostly similar to those described by Vargas and Castillo. We observed intermediate to high heterogeneous SIs on all pulse sequences with marked or moderate enhancement following intravenous gadolinium contrast media injection in two of three cavernous hemangiomas. These findings are very similar to those observed in organizing maxillary sinus hematomas and sinonasal angiomatous polyps (19, 20). Organizing sinonasal hematomas and sinonasal angiomatous polyps have a mixture of marked heterogeneous hypo-intensity and iso-intensity on MRI, surrounded by a hypo-intense peripheral rim. These findings reflect the histological heterogeneity of the lesion, which is comprised of hemorrhage, fibrosis, and neovascularization (19, 20). Sinonasal cavernous hemangiomas, organizing hematomas, and sinonasal angiomatous polyps are very similar clinically and radiologically, and some investigators, such as Wang et al. (20) and Yagisawa et al. (21), insist that these three entities are manifestations of a single disease. However, other investigators, such as Kim et al. (19), assert that sinonasal organizing hematomas and cavernous hemangiomas are distinct entities because the vascular lumina of cavernous hemangiomas are usually histologically larger than those of organizing hematomas. However, Kim et al. also raised the possibility that sinonasal angiomatous polyps and organizing hematomas represent the same entity due to similar reported CT and MR features of these two entities. In the study by Kim et al., four of 12 organizing hematomas developed within inflammatory sinonasal polyps, and they postulated that an organizing hematoma associated with a sinonasal polyp may be a special form of angiomatous polyp, though the pathogenesis is not clearly understood (19).
The two most common clinical findings of nasal hemangiomas in our study were recurrent epistaxis and nasal obstruction, which agrees with the literature (3). The clinical presentation is thought to result primarily from the mass effect but varies by lesion site.
In our study as well as in prior reports, capillary and lobular capillary hemangiomas manifested clinically and radiologically (on images without contrast enhancement) identical to ostiomeatal polyps, with the exception of benign-bone remodeling (1). When an isolated nasal mass is found, particularly with concurrent epistaxis, the radiologist should consider a vascular lesion, as well as more common polyps, in the differential diagnosis (1). In addition, an apparently benign bone change with recurrent epistaxis and a hemorrhagic nasal mass in the nasal cavity should provoke the radiologist to consider a mass of vascular origin (1). The differential diagnosis of these lesions includes juvenile angiofibroma, hemangiopericytoma, lymphoma, inverted papilloma, and melanoma, among others (1). Benign cavernous sinonasal hemangiomas and capillary hemangiomas > 2 cm can cause rather substantial bone erosion, and the CT findings can mislead the radiologist to a false diagnosis of a malignant tumor (4). Fortunately, the bone erosion in hemangiomas is rather smooth and benign looking, not aggressive or destructive, and is not associated with adjacent tissue invasion likely seen in carcinoma.
Knowledge of the characteristic imaging findings of nasal hemangiomas is important for the differential diagnosis and surgical excision planning. The traditional treatment for sinonasal hemangiomas is surgical excision via an open approach. However, recent advances in endoscopic surgical techniques have enabled endoscopic surgical resection of sinonasal hemangiomas (2, 22). In cases of capillary or lobular capillary hemangiomas, which are true tumors usually presenting as sessile or polypoid red or purple masses with arterial feeders, meticulous attention is required for the surgeon to resect the stalk without damaging the arterial feeder. In contrast, in cases of cavernous hemangiomas, which are a venous malformation composed of dilated cavernous venous structures, a relatively larger tumor size can make the surgeon more likely to choose endoscopic piecemeal resection rather than endoscopic en bloc resection with preoperative tumor embolization (22).
We cannot exclude potential selection bias in our study. Although this was a relative large series of patients diagnosed with sinonasal hemangiomas gathered over a long period, the sample size was small because of disease rarity. Another limitation was that we could not re-evaluate the histological findings because pathological specimens were not available for some of the older cases.
In summary, CT and MRI findings differed according to the two histological types of hemangiomas. Capillary and lobular hemangiomas appeared as well-circumscribed masses with intense enhancement in the early phase, which dissipated in the delayed phase. Cavernous hemangiomas, which were usually larger than capillary hemangiomas, appeared as inhomogeneous enhancing masses with heterogeneous SIs on T2-weighted images. Bony changes in all types of nasal hemangiomas correlated with tumor size. Familiarity with the characteristic imaging findings of nasal hemangiomas could aid in the pre-operative diagnosis and planning of surgical tumor excision.
Figures and Tables
Fig. 1
Computed tomography (CT) image of 59-year-old woman who presented with nasal congestion. Right nasal tumor was diagnosed as lobular capillary hemangioma.
A. Axial early-phase enhanced CT image reveals well-defined round mass with marked enhancement in right anterior nasal cavity (white arrow). B. Coronal delayed-phase enhanced CT image shows dissipation of tumor enhancement. Tumor reveals mild enhancement without adjacent bony changes (black arrow).
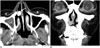
Fig. 2
Computed tomography (CT) and magnetic resonance images of 34-year-old man with right nasal tumor diagnosed as cavernous hemangioma.
A. Early-phase CT image shows mild heterogeneous enhancing tumor (arrow) in right middle meatus. B. Tumor has mild heterogeneous enhancement on coronal delayed-phase image (arrow). Coronal CT image shows erosion of right middle turbinate and lateral nasal wall. C. Mass has heterogeneous high and low signal intensity on T2-weighted axial image (arrow). D. Mass appears with iso-signal intensity (arrow) on pre-contrast T1-weighted axial image. High signal intensity area on tumor margin of T1-weighted image suggests thrombus or thick mucus retention. E. Mass has partial heterogeneous mild enhancement (arrow) on post-contrast T1-weighted axial image.
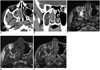
Fig. 3
Magnetic resonance imaging (MRI) of 31-year-old woman who presented with recurrent right nasal hemorrhage and nasal congestion. Nasal tumor was histologically diagnosed as capillary hemangioma.
A. Axial T1-weighted image reveals lobulating contoured mass in right anterior nasal cavity and middle meatus (white arrow). Mass has low signal intensity on T1-weighted image. Peritumoral cystic region in tumor periphery has low signal intensity on T1-weighted image (empty arrowhead). B. Right nasal mass has heterogeneously high signal intensity (large arrow) on axial T2-weighted image. There are irregular signal voids within mass (small arrows). Peritumoral cystic region has high signal intensity on T2-weighted image (empty arrowhead). C. Axial T1-weighted contrast-enhanced MRI shows markedly enhanced mass (white arrow) displacing right middle turbinate and deviating nasal septum leftward. Peritumoral cystic region exhibits no contrast enhancement (empty arrowhead).
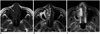
Table 1
Patients' Demographic Features, Initial Symptoms, Available Images, Size, and Contour
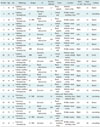
Table 2
CT and MRI Imaging Findings of Capillary/Lobular Capillary Hemangiomas and Cavernous Hemangiomas

References
1. Dillon WP, Som PM, Rosenau W. Hemangioma of the nasal vault: MR and CT features. Radiology. 1991; 180:761–765.
2. Iwata N, Hattori K, Nakagawa T, Tsujimura T. Hemangioma of the nasal cavity: a clinicopathologic study. Auris Nasus Larynx. 2002; 29:335–339.
3. Osborn DA. Haemangiomas of the nose. J Laryngol Otol. 1959; 73:174–179.
4. Kim HJ, Kim JH, Kim JH, Hwang EG. Bone erosion caused by sinonasal cavernous hemangioma: CT findings in two patients. AJNR Am J Neuroradiol. 1995; 16:1176–1178.
5. Lee DG, Lee SK, Chang HW, Kim JY, Lee HJ, Lee SM, et al. CT features of lobular capillary hemangioma of the nasal cavity. AJNR Am J Neuroradiol. 2010; 31:749–754.
6. Yang BT, Li SP, Wang YZ, Dong JY, Wang ZC. Routine and dynamic MR imaging study of lobular capillary hemangioma of the nasal cavity with comparison to inverting papilloma. AJNR Am J Neuroradiol. 2013; 34:2202–2207.
7. Feingold M. Picture of the month. Hemangioma and lymphangioma of the nose. Am J Dis Child. 1985; 139:319–332.
8. Batsakis JG, Rice DH. The pathology of head and neck tumors: vasoformative tumors, part 9A. Head Neck Surg. 1981; 3:231–239.
9. Jones JE, Nguyen A, Tabaee A. Pyogenic granuloma (pregnancy tumor) of the nasal cavity. A case report. J Reprod Med. 2000; 45:749–753.
10. el-Sayed Y, al-Serhani A. Lobular capillary haemangioma (pyogenic granuloma) of the nose. J Laryngol Otol. 1997; 111:941–945.
11. Ash JE, Old JW. Hemangiomas of the nasal septum. Trans Am Acad Ophthalmol Otolaryngol. 1950; 54:350–356.
12. Bhattacharyya N, Wenokur RK, Goodman ML. Endoscopic excision of a giant pyogenic granuloma of the nasal cavity caused by nasal packing. Rhinology. 1997; 35:44–45.
13. Lee HM, Lee SH, Hwang SJ. A giant pyogenic granuloma in the nasal cavity caused by nasal packing. Eur Arch Otorhinolaryngol. 2002; 259:231–233.
14. Miller FR, D’Agostino MA, Schlack K. Lobular capillary hemangioma of the nasal cavity. Otolaryngol Head Neck Surg. 1999; 120:783–784.
15. Lance E, Schatz C, Nach R, Thomas P. Pyogenic granuloma gravidarum of the nasal fossa: CT features. J Comput Assist Tomogr. 1992; 16:663–664.
16. Berenguer B, Mulliken JB, Enjolras O, Boon LM, Wassef M, Josset P, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003; 6:495–510.
17. Dufour H, Fesselet J, Métellus P, Figarella-Branger D, Grisoli F. Cavernous hemangioma of the sphenoid sinus: case report and review of the literature. Surg Neurol. 2001; 55:169–173.
18. Vargas MC, Castillo M. Sinonasal cavernous haemangioma: a case report. Dentomaxillofac Radiol. 2012; 41:340–341.
19. Kim EY, Kim HJ, Chung SK, Dhong HJ, Kim HY, Yim YJ, et al. Sinonasal organized hematoma: CT and MR imaging findings. AJNR Am J Neuroradiol. 2008; 29:1204–1208.
20. Wang YZ, Yang BT, Wang ZC, Song L, Xian JF. MR evaluation of sinonasal angiomatous polyp. AJNR Am J Neuroradiol. 2012; 33:767–772.
21. Yagisawa M, Ishitoya J, Tsukuda M. Hematoma-like mass of the maxillary sinus. Acta Otolaryngol. 2006; 126:277–281.
22. Song CE, Cho JH, Kim SY, Kim SW, Kim BG, Kang JM. Endoscopic resection of haemangiomas in the sinonasal cavity. J Laryngol Otol. 2009; 123:868–872.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download