Abstract
Since the introduction of pancreas transplantation more than 40 years ago, surgical techniques and immunosuppressive regiments have improved and both have contributed to increase the number and success rate of this procedure. However, graft survival corresponds to early diagnosis of organ-related complications. Thus, knowledge of the transplantation procedure and postoperative image anatomy are basic requirements for radiologists. In this article, we demonstrate the imaging spectrum of pancreas transplantation with enteric exocrine drainage.
Since the first pancreas transplantation was performed successfully with a kidney graft in 1966 (1) it has proven to be the most effective therapy for treating type I diabetes mellitus, as it achieves a non-insulin dependent normoglycemic state and reduces the incidence of severity of secondary complications. From December 1996 to December 2010, more than 37000 pancreas transplantations have been reported to the International Pancreas Transplant Registry (http://surgery.arizona.edu/).
Various transplantation procedures exist, typically including a whole cadaveric graft with a duodenal segment. The surgical anatomy of the procedure may appear complicated to radiologists unfamiliar with the surgical technique of pancreas transplantation. However, graft survival corresponds to early diagnosis of organ-related complications. Thus, knowledge of the transplantation procedure and postoperative image anatomy are basic requirements for radiologists. In this article, we will demonstrate the imaging spectrum after pancreas transplantation with enteric exocrine drainage.
The surgical techniques are diverse, and no standard methodology is used by all programs. In our institution, all procedures are performed with enteric drainage of the pancreatic graft, and the native pancreas is not removed.
Several vessels are ligated during organ procurement, including the proximal gastroduodenal artery, proximal splenic artery, proximal portal vein, superior and inferior mesenteric veins at the mesenteric root and lower rim of the pancreas, proximal superior mesenteric artery (SMA) distal to the origin of the inferior pancreaticoduodenal artery including the proximal vascular root of the mesentery, and the splenic vascular pedicle at the pancreatic tail.
Pancreatic transplant grafts are typically placed in the right lower intraperitoneal cavity or pelvis, with or without simultaneous kidney transplantation. Surgical techniques have evolved over time to consider arterial inflow, venous out-flow (endocrine secretions), and pancreatic duct exocrine drainage.
The pancreas graft receives its arterial blood supply from the SMA supplying the pancreatic head, and the splenic artery supplying the body and tail. After harvesting the allograft from the donor with donor duodenum and vascular support, an arterial "Y-graft" is prepared on the "back-table" with the donor's common iliac, internal, and external iliac arteries. The donor SMA is anastomosed to the donor external iliac artery limb of the Y-graft, and the donor splenic artery is anastomosed to the donor internal iliac artery limb of the Y-graft. The common iliac artery portion is then anastomosed to the recipient's common iliac artery or external iliac artery (Figs. 1, 2).
The donor's portal vein functions as the main graft vein.
Systemic and portal are the two choices available for venous revascularization. Systemic venous revasculization commonly involves the distal inferior vena cava, right common iliac vein, or right external iliac vein (Fig. 2A). The pancreatic portal vein is anastomosed to a main tributary of the superior mesenteric vein for portal venous drainage (Fig. 2B).
The donor duodenum containing the ampulla of Vater is harvested with the pancreas. Stapling devices are usually used for both proximal and distal ends of the donor duodenum, and these radiodense devices may offer a good landmark to localize the duodenum and pancreatic head quickly (Fig. 3). All graft exocrine secretions are drained to the small bowel (enteric drainage) with a surgical side-toside anastomosis between the graft duodenum and recipient small bowel (duodenoenterostomy). Enteric-drained transplants are usually located in the mid-abdomen to the right of midline, with the head of the pancreas situated cranially. It might be located caudally depending on the feasible distance between donor graft and recipient vessels.
The alternative for enteric drainage is bladder drainage, which is performed less often today due to disadvantages such as graft pancreatitis secondary to reflux, metabolic acidosis (bicarbonate loss from urine), and urinary tract complications such as hematuria, cystitis, or urethral complications (2).
Ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) are current modalities that contribute to the post-transplantation evaluation. Multidetector CT with a dynamic study for the pancreas graft is the most frequently requested imaging modality, as it is quick and demonstrates normal surgical anatomy including the graft, the vasculature (Figs. 4, 5), the abdominal contents, and early and late complications after transplantation. US has some advantages such as portability, lack of ionizing radiation and intravenous contrast medium, and good graft image quality due to superficial placement of the graft. Normal pancreas grafts appear hypoechoic to the surrounding omental or mesenteric fat and show homogeneous echotexture (Fig. 6) (3). However US is operator dependent, and the graft may not be visualized well due to adjacent bowel gas or overlying postoperative changes, and there may be a suboptimal sonographic window, particularly when a survey of the entire abdomen is desired. MRI can also be used to evaluate the graft including vascular complications (4) and the whole abdomen; however, it is less frequently requested by our physicians due to lack of portability and the time consumed to image the patient.
Focal edematous swelling of the donor's remaining mesenteric fat attached to graft vessels may occur and should not be misdiagnosed as focal edematous pancreatitis (5).
Venous and arterial thrombosis is one of the most serious vascular complications, which may result in pancreatic graft necrosis and subsequent graft pancreatectomy (Fig. 7). Both a venous and arterial thrombus can occur but venous thrombosis is more common. Venous stump thrombosis can propagate proximally and interfere with graft venous drainage, whereas isolated thrombosis in the splenic artery stump or distal SMA can be incidentally found as they do not contribute to graft perfusion (Figs. 8, 9) (6). Kinking of the Y-graft occasionally occurs as in enterically drained pancreatic grafts; these vessels need to be longer due to the higher positioned pancreatic grafts (Fig. 10). However it may not necessarily result in compromised blood supply to the graft pancreas (Fig. 10C). Angioplasty is considered only when there is significant hemodynamic impairment concomitant with graft dysfunction (4, 7). It has also been reported that global perfusion of the pancreatic graft and sufficient graft function is sustained by a complex system of intraparenchymal anastomosis after thrombotic occlusion of one branch of the Y-graft (7).
Rejection, as a result of alloimmunity or autoimmune recurrence, is a common cause of pancreatic graft failure. It may be either hyperacute, acute, or chronic, depending on when it occurs after transplantation. However, both acute rejection and technical failures may present with a similar clinical picture, which is often characterized by vascular thrombosis (8), and the image presentation of pancreatic rejection almost always lacks specificity and may be indistinguishable from other conditions such as pancreatitis. Serum markers such as amylase and glucose concentration lack accuracy for diagnosing rejection (9, 10). Biopsy is the only definite test to distinguish these conditions (11). In addition, a pathological assessment of the overall status of the exocrine, endocrine, and vascular components provides invaluable information with regard to graft prognosis. One of our patients with biopsy-proven mild rejection of a graft 1 year after transplantation and a follow-up abdominal CT 3 years after transplantation showed significant atrophy of the graft but presented with normal endocrine function (Fig. 11).
Transplant pancreatitis occurs to some degree in all patients postoperatively, often because of ischemia, handling, and reperfusion injury related to impaired microcirculation. It is known as self-limited edematous pancreatitis and typically involves the entire graft. A temporary elevation in serum amylase for 48-96 hours after transplantation is common, which is transient and mild, and usually without significant clinical consequence (14).
Grafts with early or mild clinical pancreatitis may be normal in appearance. However in more severe episodes, the grafts typically appear with heterogeneous echogenicity and echotexture under US and heterogeneous post-contrast enhancement on CT or MRI.
Pancreatitis may also be a result of vascular complications or rejection. Necrotizing pancreatitis is the most severe form and may result in graft pancreatectomy (Fig. 7).
Intra-abdominal hemorrhage may occur after any intraabdominal surgery, which is one of the most common reasons for repeat laparotomy (Fig. 13). Gastrointestinal bleeding is multifactorial, and infection, ulceration, rejection, and ischemia have been implicated. Perioperative anticoagulation and bleeding from the suture line of the duodenoenteric anastomosis are also possibilities causing gastrointestinal bleeding in the enteric drained pancreas (Fig. 14) (14).
A variety of bowel-related complications may occur after pancreatic transplantation, including a duodenal leak or perforation, intra-abdominal abscess, small bowel obstruction, or infectious colitis due to immunosuppression (Fig. 15). Serious problems such as leak and abscess usually occur 1-6 months after transplantation. Patients may present with fever, abdominal discomfort, and leukocytosis.
Pancreatic leakage is rarely encountered, as most procedures are performed with enteric drainage for exocrine secretions. However, the complication might occur when the graft pancreas is biopsied (Fig. 16).
Post-transplantation lymphoproliferative disorder is a rare but serious complication of pancreatic transplantation; however, it shows indistinguishable enlargement of the pancreatic graft from acute pancreatitis. Sometimes it presents as intra- or extra-allograft focal masses, lymphadenopathy, or organomegaly (3).
Post-transplant Kaposi's sarcoma is more common in patients on cyclosporine immunosuppression than on non-cyclosporine-based regimens and is associated with the HHV8 virus (Fig. 17) (15).
Hepatic veno-occlusive disease is defined as nonthrombotic fibrous obliterative endophlebitis of small centrilobular hepatic venules and is usually reported as a complication of stem cell and solid organ transplantation. It is considered in association with the hepatotoxicity of cyclosporine or tacrolimus (16). Patients clinically present with elevated liver enzymes, jaundice, hepatomegaly, and ascites (Fig. 18). The toxicity is reversible with cessation or reduction of tacrolimus.
Imaging and interpretation of an entire pancreatic transplantation requires a basic understanding of surgical anatomy. CT and US are good techniques for postoperative diagnostic imaging and each have their advantages and disadvantages. CT is the most frequently requested imaging modality, as it is quick and demonstrates general postoperative intra-abdominal conditions including early and late complications after transplantation.
Figures and Tables
Fig. 1
Pancreas is typically placed in right lower intraperitoneal cavity or pelvis, and kidney is placed on left during simultaneous pancreas-kidney transplantation. Common iliac artery portion of Y-graft (red arrowhead) is anastomosed to recipient's common iliac artery or external iliac artery. Donor superior mesenteric vein (black arrowhead) is anastomosed to distal inferior vena cava in systemic drainage (curved arrow, donor duodenum; black star, pancreas; red star, kidney; arrows, renal vessels).
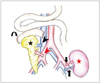
Fig. 2
Illustration of pancreas transplantation alone.
A. With systemic venous revasculization (arrow, anastomosis of graft superior mesenteric vein [SMV] to inferior vena cava [IVC]; arrowhead, anastomosis of donor Y-graft to common or external iliac artery). B. With portal venous revascularization (arrow, anastomosis of graft SMV to major branch of recipient SMV). Both procedures are performed with enteric exocrine drainage (curved arrow, donor duodenum; black star, pancreas).
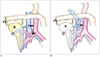
Fig. 3
Stapling device. Stapling devices are usually used for both proximal and distal ends of donor duodenum, and these radiodense devices may offer good landmarks to localize duodenum and pancreatic head quickly on computed tomography scan (white arrow, surgical staples; star, pancreas head).
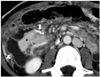
Fig. 4
Anatomy of Y-graft with two limbs anastomosed with superior mesenteric vein and splenic artery. After harvesting allograft from donor, arterial "Y-graft" is prepared on "back-table" with donor common iliac, internal, and external iliac arteries. Donor superior mesenteric artery (SMA) is anastomosed to donor external iliac artery limb of Y-graft, and donor splenic artery is anastomosed to donor internal iliac artery limb of Y-graft. Common iliac artery portion is then anastomosed to recipient's common iliac artery or external iliac artery.
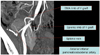
Fig. 5
Normal venous graft appearance.
A. 33-year-old man with simultaneous pancreas-kidney transplantation. Normal venous graft appearance and anastomosis of donor superior mesenteric vein (SMV) to right iliac vein (arrow) is shown on axial computed tomography (CT) view (star, graft kidney). B, C. 41-year-old man with pancreatic transplantation alone. Patent venous graft sometimes appears collapsed on CT, yet it is patent (star, pancreas; white arrow in B and black arrow in C; anastomosis of donor SMV to inferior vena cava; arrowhead, anastomosis of Y-graft to recipient right iliac artery).

Fig. 6
Normal pancreatic grafts appear hypoechoic to surrounding omental or mesenteric fat and show homogeneous echotexture (white arrowheads).
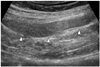
Fig. 7
51-year-old male with pancreas necrosis 2 weeks after simultaneous pancreas-kidney transplantation. Computed tomography shows absence of parenchymal enhancement, diffuse enlargement of graft wrapping around graft kidney, and poor graft vascular opacification (white arrows, necrotic pancreas, curved arrow, surgical staple line). He finally received graft pancreatectomy. Note that graft kidney (black star) is normal.
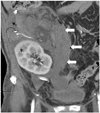
Fig. 8
29-year-old female after simultaneous pancreas-kidney transplantation with thrombosis in donor splenic vein and superior mesenteric vein.
A. Non-contrast axial computed tomography (CT) scan was performed 8 days after operation (arrows, thrombus). B. Contrast-enhanced CT was performed 3 weeks after operation. Persistent thrombosis within donor veins was seen (white arrows, thrombus; black triangles, pancreas; black star, kidney).

Fig. 9
31-year-old female 11 days after pancreatic transplantation.
A. Computed tomography scan shows venous thrombosis (arrow, filling defect in donor splenic vein; white star, pancreas). B. Angiography confirmed presence of thrombus in donor splenic vein. However, endocrine function of graft pancreas was clinically normal. Increased collateral venous network had developed (black arrows), which probably preserved pancreatic graft from venous thrombotic pancreatitis (white arrow, thrombus in donor splenic vein).
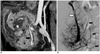
Fig. 10
31-year-old female 11 days after pancreatic transplantation.
Maximal intensity projection image from computed tomography angiography (A) shows kinking of Y-graft, which was confirmed on angiography (B). However, blood supply to both limbs of Y-graft and endocrine function of graft were normal (arrow, patent Y-graft; arrowheads, kinking of both limbs; star, kidney). C. Superior mesenteric artery limb and splenic artery limb showed progressive narrowing 1 year later, yet perfusion and endocrine function of graft pancreas remained normal. Angioplasty is considered only when there is significant hemodynamic impairment concomitant with graft dysfunction (arrow, kinking sites with narrowing; star, pancreatic head).
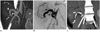
Fig. 11
20-year-old male after pancreatic transplantation alone.
One year after operation (A) computed tomography (CT) scan shows mild swelling of pancreas (star) and (B) ultrasound-guided biopsy proved mild rejection. Swelling of pancreas with hypoechoic echogenicity was noted (white star). C. Follow-up CT scan 3 years after operation showed significant atrophy of pancreas. However, endocrine function of graft pancreas was normal (arrowheads, atrophic pancreas; arrow, donor duodenum with surgical staples).

Fig. 12
37-year-old female with pancreatic transplantation alone.
She presented with markedly elevated pancreatic enzymes 16 months after operation. A. Contrast-enhanced computed tomography (CT) showed swelling of pancreatic head with heterogeneous contrast enhancement (white arrows) and duodenal wall edema (black arrow) indicating pancreatitis. B. Gray-scale ultrasound also revealed swollen hypoechoic pancreatic head (white arrows) and biopsy proven acute rejection. We cannot distinguish rejection from pancreatitis on contrast-enhanced CT scan or ultrasound images, and biopsy is only way to confirm this diagnosis.
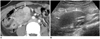
Fig. 13
43-year-old male 1 day after simultaneous pancreas-kidney transplantation. Hematoma was seen on non-contrast computed tomography scan (star, pancreas; arrows, hematoma). He underwent exploratory laparotomy to check for bleeder and to remove hematoma.
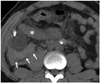
Fig. 14
34-year-old female 2 days after pancreatic transplantation alone. Computed tomography scan showed active bleeding (arrow) of Roux-limb of intestine. Bleeding subsided after conservative treatment.
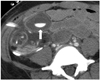
Fig. 15
30-year-old male after pancreatic transplantation alone. Intestinal obstruction with adhesion band is noted 8 months after operation (arrows, fluid-filled dilated bowel loops; star, pancreas).
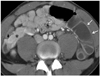
Fig. 16
26-year-old female after pancreatic transplantation alone. Biopsy was performed intraoperatively because donor had systemic lupus erythematosus. Computed tomography scan 1 week after operation showed fluid collection around graft pancreas (arrow) and exploratory laparotomy revealed pancreatic juice leakage from biopsy site.
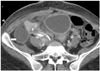
Fig. 17
33-year-old male after pancreatic transplantation alone.
Multiple soft tissue nodules in mediastinum (A, black arrow) and abdomen (B, black arrow) are noted on contrast-enhanced computed tomography 15 months after transplantation. Biopsy of neck lymph nodes proved Kaposi's sarcoma and HHV-8 positivity.
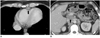
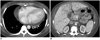
Fig. 18
25-year-old female after simultaneous pancreas-kidney transplantation with hepatic veno-occlusive disease.
Patient presented 9 months after operation with elevated liver enzymes and jaundice. Contrast-enhanced computed tomography (CT) scan showed pericardial effusion (A), hepatomegaly with heterogeneous race-like enhancement of hepatic parenchyma, periportal edema, and ascites (B). Surgeon shifted her immunosuppressant from tacrolimus to cyclosporine, and CT after 3 months revealed significant resolution of abnormal findings seen in previous study.
Acknowledgments
We thank the other staffs of the Department of Radiology of Taipei Veterans General Hospital for their valuable contributions.
References
1. Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967; 61:827–837.
2. Baktavatsalam R, Little DM, Connolly EM, Farrell JG, Hickey DP. Complications relating to the urinary tract associated with bladder-drained pancreatic transplantation. Br J Urol. 1998; 81:219–223.
3. Vandermeer FQ, Manning MA, Frazier AA, Wong-You-Cheong JJ. Imaging of whole-organ pancreas transplants. Radiographics. 2012; 32:411–435.
4. Hagspiel KD, Nandalur K, Pruett TL, Leung DA, Angle JF, Spinosa DJ, et al. Evaluation of vascular complications of pancreas transplantation with high-spatial-resolution contrast-enhanced MR angiography. Radiology. 2007; 242:590–599.
5. Freund MC, Steurer W, Gassner EM, Unsinn KM, Rieger M, Koenigsrainer A, et al. Spectrum of imaging findings after pancreas transplantation with enteric exocrine drainage: Part 2, posttransplantation complications. AJR Am J Roentgenol. 2004; 182:919–925.
6. Chandra J, Phillips RR, Boardman P, Gleeson FV, Anderson EM. Pancreas transplants. Clin Radiol. 2009; 64:714–723.
7. Margreiter C, Mark W, Wiedemann D, Sucher R, Ollinger R, Bösmüller C, et al. Pancreatic graft survival despite partial vascular graft thrombosis due to splenocephalic anastomoses. Am J Transplant. 2010; 10:846–851.
8. Drachenberg CB, Papadimitriou JC. Spectrum of histopathological changes in pancreas allograft biopsies and relationship to graft loss. Transplant Proc. 2007; 39:2326–2328.
9. Holalkere NS, Soto J. Imaging of miscellaneous pancreatic pathology (trauma, transplant, infections, and deposition). Radiol Clin North Am. 2012; 50:515–528.
10. Dillman JR, Elsayes KM, Bude RO, Platt JF, Francis IR. Imaging of pancreas transplants: postoperative findings with clinical correlation. J Comput Assist Tomogr. 2009; 33:609–617.
11. Atwell TD, Gorman B, Larson TS, Charboneau JW, Ingalls Hanson BM, Stegall MD. Pancreas transplants: experience with 232 percutaneous US-guided biopsy procedures in 88 patients. Radiology. 2004; 231:845–849.
12. Nelson NL, Largen PS, Stratta RJ, Taylor RJ, Grune MT, Hapke MR, et al. Pancreas allograft rejection: correlation of transduodenal core biopsy with Doppler resistive index. Radiology. 1996; 200:91–94.
13. Aideyan OA, Foshager MC, Benedetti E, Troppmann C, Gruessner RW. Correlation of the arterial resistive index in pancreas transplants of patients with transplant rejection. AJR Am J Roentgenol. 1997; 168:1445–1447.
14. Shyr YM, Su CH, Li AF, Wu CW, Lui WY. Canine pancreas allotransplantation with enteric drainage. Zhonghua Yi Xue Za Zhi (Taipei). 2002; 65:483–488.
15. Farge D. Kaposi’s sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur J Med. 1993; 2:339–334.
16. Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic venoocclusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003; 10:451–462.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download