Abstract
Recent progress in chemotherapy has prolonged the survival of patients with malignant biliary strictures, leading to increased rates of stent occlusion. Even we employed metallic stents which contributed to higher rates and longer durations of patency, and occlusion of covered metallic stents now occurs in about half of all patients during their survival. We investigated the complication and patency rate for the removal of covered metallic stents, and found that the durations were similar for initial stent placement and re-intervention. In order to preserve patient quality of life, we currently recommend the use of covered metallic stents for patients with malignant biliary obstruction because of their removability and longest patency duration, even though uncovered metallic stents have similar patency durations.
Since the initial report by Soehendra et al. (1), the outcomes of endoscopic retrograde biliary drainage have improved considerably with the development of large-bore, metallic, and covered metallic stents. However, this improvement has contributed to higher rates and longer patency durations (2-11). Recent progress in chemotherapy has prolonged the survival of patients with unresectable malignant tumors associated with biliary obstruction (12-15). The patency period of even metallic stents is shorter than required, and about half of all patients now require the placement of 2 or 3 stents during their lifetime. Many studies concerning the management of occluded or dislocated biliary stents have been reported (16-22). Mechanical cleaning with a balloon and "stent-in-stent" placement with a plastic tube or other devices have been performed, but the patency duration was rather short. The removal of metallic stents has been sporadically reported, suggesting that stent removal followed by replacement may be one way to manage occluded or dislocated stents (23-29). We investigated the patency durations and rates of the first stent as well as re-intervention, success rates of stent removal, and complications, especially for the management of patients with malignant biliary obstruction who have survived more than the stent patency duration.
Remarkable progress has been recently made in the chemotherapy and radiotherapy of unresectable malignant tumors of the biliary tract and pancreas, which has led to increasing numbers of long-term survivors. Although the patency duration of metallic stents has been prolonged, the patency duration of a single stent is often too short, and the number of patients who clinically require the placement of second or third metallic stents is increasing. According to our data, the stent-occlusion rate was 36.0% (67/186) overall and 49.0% (67/137) among the survivors. Thus, nearly half of all surviving patients had a stent occlusion. The main causes of stent occlusion were debris or food residue (25 patients, 37%), dislocation (13 patients, 19%), and migration with hyperplasia (13 patients, 19%) (Tables 1, 2) (35).
Stent occlusion due to debris or food residue is usually treated by lavage with a balloon-tipped catheter or a "stent-in-stent" procedure. Previous studies reported that the stent patency duration was 21 to 34 days after mechanical lavage with a balloon-tipped catheter, 90 days after the placement of a plastic stent in an self-expandable metal stent (SEMS), and 75 to 192 days after a "stent-in-stent" placement of a SEMS (17, 18). The patency duration after these procedures was slightly shorter than after placement of the initial SEMS. However, because SEMSs have the longest patency duration, they are considered clinically useful for the treatment of initial stent occlusion (17, 18, 33). From the viewpoint of cost effectiveness and patient quality of life, SEMSs are also recommended as the second stent (34).
Ideally, the first metallic stent should be removed and then replaced by an SEMS. In previous studies, stent removal was performed only if a stent was misplaced soon after placement. This procedure was considered risky and was unsuccessful in many patients (16). In subsequent studies, stents were removed or cut to treat complications such as ulcers or stent occlusion due to migration or dislocation. In the case reports, laser treatment (19), forceps (24), suture-cutting devices (26), snares (25, 28), and argon plasma coagulation (20-23) have been used to remove or cut stents.
In 2004, Kahaleh et al. (29) reported the results of stent removal in a series of 18 patients. The period of time from stent placement to removal ranged from 1 week to 16 months in 4 patients with uncovered SEMSs and 1 to 11 months in 14 with covered SEMSs. In prospective studies, Familiari et al. (31) and Shin et al. (32) reported that 0% to 38.4% of uncovered SEMSs and 86.4% to 92.3% of covered SEMSs could be removed with no serious complications.
According to our data, we have attempted metallic stent removal in 50 cases and 78.0% (39/50) of the covered SEMSs could be removed (35) (Fig. 1). Ten stents had migrated into the bile duct and had hyperplastic-tissue ingrowth of the uncovered portion at the distal end of the SEMS, and 1 stent that had remained in place for 2 years 5 months and was suspected to have tumor ingrowth and could not be removed. Our results and those of previous studies suggest that a covered Wallstent or Wallflex stent can be removed within 1.0-1.5 years after initial placement if there is no stent migration with hyperplastic-tissue ingrowth of the uncovered portion. Even stents with hyperplastic-tissue ingrowth of the uncovered portion could be removed if the stent slightly protruded towards the duodenum, allowing the protruding portion to be grasped with a snare. The hyperplastic tissue inside the stent was then incised with a needle knife, allowing the stent to be removed with the snare. Another technique so called the "inversion technique", in which the inner end of the stent is caught by forceps and removed. Futhermore, the problem of hyperplastic-tissue ingrowth may be solved if we employ a "full covered" stent.
In order to prevent stent migration, it was reported that stents one size longer than the length was required (32). We also inserted longer metallic stents measuring 6 or 8 cm in length in most patients because the axial force of a 4-cm metallic stent has caused biliary obstruction. Nonetheless, proximal migration with hyperplasia occurred in 19% of cases; however, we should emphasize that none of the second stents have shown proximal migration at the time of this writing.
Using a duodenal endoscope with a 3.7-mm channel, most stents could be removed through the channel by snaring the stent (Fig. 1). Furthermore, all stents could be removed when a duodenal endoscope with a 4.2-mm channel was used. Removal of a Wallflex stent might require the use of a duodenal endoscope with a 4.2-mm channel.
Ornellas et al. (33) compared 48 patients with initially placed covered SEMSs with 56 different patients on their second stents and found that the patency duration did not differ significantly between the groups (p = 0.057). However, the initial placement group included a significantly higher proportion of patients with advanced disease, whereas the re-intervention group included a significantly higher proportion of patients with proximal biliary strictures.
We also studied the effectiveness of primary stent placement, as well as the effectiveness and safety of secondary stent placement (re-intervention) in patients with unresectable malignant biliary obstructions (35). In our study, there was no difference in demographic characteristics between the initial placement group and the re-intervention group, and all patients in the latter group were included in the former. Because a longer time had elapsed since the diagnosis in the re-intervention group, the disease stage was more advanced than at the time of initial placement. However, demographic characteristics such as age, sex, and underlying disease were similar in these groups. The patency rates and patency durations also did not differ significantly between these groups (p = 0.08) (Fig. 2). There was also no significant difference in the patency rates and patency durations between initially placed stents and secondarily placed stents in the same patients (p = 0.07) (Fig. 3). Although the patency durations of the first stent in the removed group, where the first stents were removed and a second stent was inserted, was shorter than that of all the first stents and hence must be biased. Therefore, we also compared the patency durations of the second stents with all the first stents, and a log-rank analysis confirmed the lack of a significant difference (Figs. 2, 3).
A consensus has not been reached as to whether covered metallic stents have a significantly longer patency duration than uncovered metallic stents (7-11). Futhermore, covered metallic stents are also considered to have a significantly higher risk of migration and dislocation (9-11). However we confirmed that stents could be removed easily and safely. Given that further prolongation of the patency duration is unlikely in the foreseeable future, covered metallic stents are best suited for re-intervention because they have the longest durations of patency.
We believe that our results have important implications for clinical practice. However, additional prospective studies comparing re-interventions after the removal of metallic stents with "stent-in-stent" placement of metallic stents are needed. Studies with a larger number of patients are also necessary. At present, the covered metallic stents can be relatively easily reinserted after the removal of the initially placed covered metallic stents, without causing complications. Patency rates and durations of the second stents are similar to those of the initially placed stents. To preserve patient quality of life, we currently recommend covered metallic stents for patients with malignant biliary obstruction because of their removability and longest durations of patency, although uncovered metallic stents have similar patency durations.
Figures and Tables
Fig. 1
Distal end of Covered SEMS was caught by snare and removed through duodenal endoscope channel.
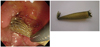
Fig. 2
Kaplan-meier curves comparing cumulative patency of first and second metallic stents in all 186 cases (Endoscopy 2011;43:1039-1044). MPT = median patency time

Fig. 3
Kaplan-meier curves comparing cumulative patency of first and second metallic stents in same patients out of 186 patients (Endoscopy 2011;43:1039-1044). MPT = median patency time

References
1. Soehendra N, Reynders-Frederix V. [Palliative biliary duct drainage. A new method for endoscopic introduction of a new drain]. Dtsch Med Wochenschr. 1979. 104:206–207.
2. Huibregtse K, Haverkamp HJ, Tytgat GN. Transpapillary positioning of a large 3.2 mm biliary endoprosthesis. Endoscopy. 1981. 13:217–219.
3. Huibregtse K, Cheng J, Coene PP, Fockens P, Tytgat GN. Endoscopic placement of expandable metal stents for biliary strictures--a preliminary report on experience with 33 patients. Endoscopy. 1989. 21:280–282.
4. Neuhaus H, Hagenmüller F, Classen M. Self-expanding biliary stents: preliminary clinical experience. Endoscopy. 1989. 21:225–228.
5. Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992. 340:1488–1492.
6. Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993. 25:207–212.
7. Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, et al. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004. 53:729–734.
8. Yoon WJ, Lee JK, Lee KH, Lee WJ, Ryu JK, Kim YT, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006. 63:996–1000.
9. Park do H, Kim MH, Choi JS, Lee SS, Seo DW, Kim JH, et al. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin Gastroenterol Hepatol. 2006. 4:790–796.
10. Telford JJ, Carr-Locke DL, Baron TH, Poneros JM, Bounds BC, Kelsey PB, et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010. 72:907–914.
11. Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010. 72:915–923.
12. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997. 15:2403–2413.
13. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007. 25:1960–1966.
14. Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009. 101:621–627.
15. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010. 362:1273–1281.
16. Schöfl R, Brownstone E, Reichel W, Fortunat W, Doblhofer F, Samec HJ, et al. Malignant bile-duct obstruction: experience with self-expanding metal endoprostheses (Wallstents) in Austria. Endoscopy. 1994. 26:592–596.
17. Tham TC, Carr-Locke DL, Vandervoort J, Wong RC, Lichtenstein DR, Van Dam J, et al. Management of occluded biliary Wallstents. Gut. 1998. 42:703–707.
18. Bueno JT, Gerdes H, Kurtz RC. Endoscopic management of occluded biliary Wallstents: a cancer center experience. Gastrointest Endosc. 2003. 58:879–884.
19. Yarze JC, Poulos AM, Fritz HP, Herlihy KJ. Treatment of metallic biliary stent-induced duodenal ulceration using endoscopic laser therapy. Dig Dis Sci. 1997. 42:6–9.
20. Demarquay JF, Dumas R, Peten EP, Rampal P. Argon plasma endoscopic section of biliary metallic prostheses. Endoscopy. 2001. 33:289–290.
21. Vanbiervliet G, Piche T, Caroli-Bosc FX, Dumas R, Peten EP, Huet PM, et al. Endoscopic argon plasma trimming of biliary and gastrointestinal metallic stents. Endoscopy. 2005. 37:434–438.
22. Chen YK, Jakribettuu V, Springer EW, Shah RJ, Penberthy J, Nash SR. Safety and efficacy of argon plasma coagulation trimming of malpositioned and migrated biliary metal stents: a controlled study in the porcine model. Am J Gastroenterol. 2006. 101:2025–2030.
23. Rerknimitr R, Naprasert P, Kongkam P, Kullavanijaya P. Trimming a metallic biliary stent using an argon plasma coagulator. Cardiovasc Intervent Radiol. 2007. 30:534–536.
24. Ahmed A, Keeffe EB, Imperial JC. A novel technique for endoscopic removal of expandable biliary Wallstent. Gastrointest Endosc. 1999. 50:279–281.
25. Egan LJ, Baron TH. Endoscopic removal of an embedded biliary Wallstent by piecemeal extraction. Endoscopy. 2000. 32:492–494.
26. Levy MJ, Wiersema MJ. Endoscopic removal of a biliary Wallstent with a suture-cutting device in a patient with primary pancreatic lymphoma. Endoscopy. 2002. 34:835–837.
27. Wamsteker EJ, Elta GH. Migration of covered biliary self-expanding metallic stents in two patients with malignant biliary obstruction. Gastrointest Endosc. 2003. 58:792–793.
28. Trentino P, Falasco G, d'orta C, Coda S. Endoscopic removal of a metallic biliary stent: case report. Gastrointest Endosc. 2004. 59:321–323.
29. Kahaleh M, Tokar J, Le T, Yeaton P. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004. 60:640–644.
30. Costamagna G, Pandolfi M. Endoscopic stenting for biliary and pancreatic malignancies. J Clin Gastroenterol. 2004. 38:59–67.
31. Familiari P, Bulajic M, Mutignani M, Lee LS, Spera G, Spada C, et al. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005. 62:903–910.
32. Shin HP, Kim MH, Jung SW, Kim JC, Choi EK, Han J, et al. Endoscopic removal of biliary self-expandable metallic stents: a prospective study. Endoscopy. 2006. 38:1250–1255.
33. Ornellas LC, Stefanidis G, Chuttani R, Gelrud A, Kelleher TB, Pleskow DK. Covered Wallstents for palliation of malignant biliary obstruction: primary stent placement versus reintervention. Gastrointest Endosc. 2009. 70:676–683.
34. Arguedas MR, Heudebert GH, Stinnett AA, Wilcox CM. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: a cost-effectiveness analysis. Am J Gastroenterol. 2002. 97:898–904.
35. Kida M, Miyazawa S, Iwai T, Ikeda H, Takezawa M, Kikuchi H, et al. Endoscopic management of malignant biliary obstruction by means of covered metallic stents: primary stent placement vs. re-intervention. Endoscopy. 2011. 43:1039–1104.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download