Abstract
An imaging-guided core needle biopsy has been proven to be reliable and accurate for the diagnosis of both benign and malignant diseases of the breast, and has replaced surgical biopsy. However, the possibility of a false-negative biopsy still remains. Imaging-pathology correlation is of critical importance in imaging-guided breast biopsies to detect such a possible sampling error and avoid a delay in diagnosis. We will review five possible categories and corresponding management after performing an imaging-pathology correlation in a sonography-guided core needle biopsy of a breast lesion, as well as illustrate the selected images for each category in conjunction with the pathologic finding. Radiologists should be familiar with the imaging features of various breast pathologies and be able to appropriately correlate imaging findings with pathologic results after a core needle biopsy.
The success of an imaging-guided core needle breast biopsy depends on the post-biopsy management as well as the performance of the biopsy procedure (1). Any core biopsy procedure may fail to sample a cancer, resulting in a benign, often nonspecific pathologic diagnosis, despite optimization of the technique (2). Although there are methods such as specimen radiography or post-biopsy mammography to confirm lesion retrieval after performing a core biopsy, they often provide incomplete information, especially in lesions visualized by sonography only. Correlation of the pathologic result with the imaging findings after biopsy is found to be useful to validate the biopsy result and to offer subsequent management (2-6).
The purpose of this study is to review derived categories and corresponding management for an imaging-pathology correlation after performing a sonography-guided core needle biopsy and to illustrate the selected images for each category, which will provide guidance in the application of this post-biopsy assessment in practice.
Before beginning the biopsy procedure, all imaging features of a targeted lesion, mammography, sonography, and MRI, should be carefully reviewed. Moreover, the diagnosis criteria for the finding in question as well as setting the probability of malignancy based on these set criteria should be predetermined (7). When the pathology result is received after biopsy, the radiologist can compare the pathologic diagnosis with the expected result from the imaging finding. Images documented during the biopsy should also be carefully reviewed to verify whether the lesion was accurately targeted with regard to sampling the wrong lesion, suboptimal sampling (i.e., patient movement or insufficient penetration), and procedural complications (i.e., bleeding or hematoma formation) (1, 4, 7). A strong working relationship between the radiologist and pathologist is important for imaging-pathology correlation. The pathologist is critical in assessing and communicating the quantitative and qualitative aspects of the biopsy (1, 4).
The imaging and pathologic findings are considered to be concordant when the pathologic result provides an acceptable explanation for the imaging feature and discordant when they do not. After the assessment for concordance has been completed, a management plan can be provided. Parikh and Tickman (4) described five possible outcomes of imaging-pathology correlation and suggested corresponding management for each category.
A lesion which showed a suspicious finding for malignancy on images (i.e., Breast Imaging Reporting and Data System [BI-RADS] category 4 or 5) and is diagnosed to be malignant on a subsequent core needle biopsy is a concordant malignancy (4) (Fig. 1). Within this category, appropriate action should be taken without any delay. The radiologist should communicate the biopsy result to the referring physician, and the patient should be informed of the results and referred to a surgeon or oncologist for proper treatment.
A lesion which typically had benign or benign-favoring imaging features (i.e., BI-RADS category 2 or 3) but proves to be malignant at core needle biopsy falls into this category (Fig. 2). Case management should be identical to that for a concordant malignancy (2, 4). Lesions that usually lie at the circumscribed end of the malignant spectrum can simulate benign nodules; small infiltrating ductal carcinomas, high-grade invasive ductal carcinomas not otherwise specified, metastatic lesions, lymphoma, and special-type tumors that are well circumscribed such as medullary carcinomas, mucinous carcinomas, and papillary carcinoma (8). The radiologist should notify the discordant result to the pathologist and ask the patient to review and confirm the diagnosis. Also, the images of the lesion should be reviewed for image quality, lesion characteristics, and missed associated features which may cause an underestimation of the severity of the lesion. The discrepancy between imaging and pathologic results should be discussed thoroughly.
A lesion which is initially thought to be benign radiologically (i.e., BI-RADS category 2, 3, or 4a) and also demonstrates benign pathology at core needle biopsy falls into this category (2, 4) (Fig. 3). This result can offer both the physician and the patient reassurance. However, imaging follow-up should be recommended to patients because of delayed false-negative diagnoses at core biopsy (2). Although there is no standard follow-up guideline, a follow-up sonography at six months after biopsy and then annually for at least two years can be recommended (Fig. 4).
A lesion in this category is suspicious for malignancy at imaging (i.e., BI-RADS category 4 or 5), but demonstrates benign pathologic result after performing a core needle biopsy (2, 4) (Figs. 5-8). Benign lesions with spiculated findings can simulate malignant lesions and be considered as differential diagnoses as follows: sclerosing adenosis, fat necrosis, postsurgical scar, mastitis, granular cell tumor, diabetic mastopathy, and sarcoidosis (8). However, the radiologist should give special attention to discordant benign lesions from which a substantial number of missed cancers at core needle biopsy can be detected without any delay in diagnosis (Figs. 6, 7). In published reports, up to 64% of discordant lesions after a percutaneous biopsy were confirmed as cancer by subsequent surgical excision (5). For a sonography-guided 14-gauge core needle biopsy, discordant lesions had cancer rates of up to 50% (6). If there is concern regarding a discordant benign lesion, it is prudent for the radiologist to immediately contact the interpreting pathologist and thoroughly communicate with each other. According to the discussion, the radiologist should notify the result and discuss the need for a repeat biopsy to the referring physician or the patient. A surgical biopsy, rather than a core needle biopsy, is recommended for a repeat biopsy because of the inconclusive outcome from the first core biopsy. Recently, the vacuum-assisted core needle biopsy has been reported to be an alternative to surgery to obtain a definitive histological diagnosis for discordant benign lesions (9).
A lesion in this category is not malignant but is considered to have an increased lifetime risk for the development of breast cancer (e.g., atypical ductal hyperplasia, lobular neoplasm, radial sclerosing lesion, papillary lesions, possible phyllodes tumors) (2, 4) (Figs. 9, 10). Controversy exists regarding the surgical and oncologic treatment for this lesion. A case-by-case approach is needed to manage the patient in accordance with the active discussion between different subspecialties (4). However, surgical biopsy is usually recommended regardless of concordance, because of the relatively high upgrade rate to malignancy.
Since the establishment of BI-RADS for sonography in 2003, the reliability of the sonographic BI-RADS lexicon or classification in evaluating sonographic masses for the likelihood of malignancy have been assessed and reported to have a good performance (10-13). Based on the BI-RADS category, the indication for biopsy of particular lesions can be clarified.
A category 3 lesion is judged to have a 2% or lower probability of malignancy and suggested to be followed up with short-term imaging surveillance. While performing a biopsy is contradictory to a category 3 lesion, it is performed in a specific circumstance such as physician or patient preference. For a category 3 lesion, a benign core biopsy result can be regarded as concordant benign and a malignant core biopsy result as discordant malignant. However, subtle suspicious sonographic features are sometimes overlooked because the final assessment category is determined based on the individual radiologist's experience and training as well as published criteria. In imaging-pathology correlation, therefore, the sonographic features, even for category 3 lesions, should be reviewed based on the strict criteria to avoid missing cancer.
While BI-RADS category 4 lesions have been recommended for biopsy, a wide range of positive predictive value (3% to 94%) is problematic. A new recommendation in the fourth edition of BI-RADS is for category 4 to be subdivided internally into three subgroups (4a, 4b, and 4c) on the basis of the likelihood of malignancy, although optional (14). The BI-RADS did not set out specific guidelines regarding what was the risk of malignancy for each of the subcategories should represent. However, Bent et al. (15) suggested that the guidance range of malignancy likelihood should be 2-10% for category 4a, 11-50% for category 4b, and 51-95% for category 4c. In a study of categorizing lesions by mammography or sonography (11), PPV was 6%, 15%, and 53% for categories 4a, 4b, and 4c, respectively. Whereas, Lee et al. (13) reported that PPV for sonography was 26%, 83%, and 91% for category 4a, 4b, and 4c, respectively. The interobserver variability and poor stratification for the risk of malignancy in the subcategories could be explained by the lack of known factors clearly and objectively defining each subdivision. Because the use of subcategories is optional and clinical data of those subcategories are limited, management is not standardized. Based on BI-RADS (14), the benign core biopsy result may be regarded as concordant benign and the malignant core biopsy result as discordant malignant for a category 4a lesion. The benign core biopsy result may be regarded as discordant benign and the malignant core biopsy result as concordant malignant for a category 4c lesion. For category 4b lesions, close imaging and pathologic correlations are needed. Further studies are needed to evaluate the role of these subdivisions in stratifying the level of suspicion and the use of this information in management decisions.
In conclusions, careful imaging-pathology correlation and appropriated post-biopsy management should be the cornerstone of a successful core biopsy program. It will allow the detection of a substantial number of false-negative results immediately after core needle biopsy by identifying discordant lesions prospectively, thereby avoiding delays in the diagnosis of cancer. Although the communication between the radiologist and pathologist is the basis of imaging-pathology correlation, establishing concordance is subject to the experience of the radiologist. The radiologist performing the biopsy must be familiar with the imaging features of a vast array of pathologic breast lesions and they must be able to correlate with each other.
Figures and Tables
Fig. 1
46-year-old woman with palpable mass in her right breast.
A. On mammography (left: mediolateral oblique view, right: craniocaudal view), irregular ill-defined hyperdense mass (arrows) is seen on area corresponding to palpable abnormality. B. On sonography (left: transverse view, right: longitudinal view), mass is determined to be round microlobulated hypoechoic mass with nonparallel orientation and classified as BI-RADS category 4c. Result of sonography-guided 14-gauge core needle biopsy are consistent with invasive ductal carcinoma, which is considered as concordant malignancy. C. Histologic features on low power view indicate invasive ductal carcinoma with peripheral intraductal component (arrows) without microcalcification. Surrounding breast parenchyma shows fatty changes (Hematoxylin & Eosin staining; original magnification, × 10).
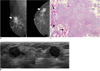
Fig. 2
46-year-old woman with mass in her left breast.
A. On mammography (left: mediolateral oblique view, right: craniocaudal view), there is focal asymmetry (arrows) in upper outer portion of left breast. B. On sonography (left: transverse view, right: longitudinal view), oval circumscribed hypoechoic mass (arrows) is seen and classified as BI-RADS category 3. Sonography-guided 14-gauge core needle biopsy was performed at request of patient and pathologic result is consistent with invasive ductal carcinoma, which is considered as discordant malignancy. C. Photomicrograph of microscopic specimen after surgical excision shows relatively well-differentiated tumor cell nests dispersed in desmoplastic stroma (arrows). No intraductal component was seen in submitted specimen. Final diagnosis was invasive ductal carcinoma (Hematoxylin & Eosin staining; original magnification, × 100).

Fig. 3
45-year-old woman with mass in her left breast.
A. On mammography (left: mediolateral oblique view, right: craniocaudal view), oval circumscribed mass (arrows) is seen in upper outer portion of her left breast. B. On sonography (left: transverse view, right: longitudinal view), oval circumscribed hypoechoic mass is seen and classified as BI-RADS category 3. Sonography-guided 14-gauge core needle biopsy was performed at request of patient and pathologic result indicated fibroadenoma, which is considered to be concordant benign lesion. However, in lieu of follow-up image, surgical excision was performed. C. Photomicrograph of microscopic specimen after surgical excision shows sharply defined border with both glandular (arrowhead) and stromal proliferation (arrow), mainly showing intracanalicular growth pattern. Final diagnosis was fibroadenoma (Hematoxylin & Eosin staining; original magnification, × 10).
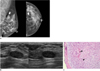
Fig. 4
56-year-old woman with palpable mass in her left breast.
A. On sonography (left: transverse view, right: longitudinal view), oval circumscribed hypoechoic mass is seen classified as BI-RADS category 3. Sonography-guided 14-gauge core needle biopsy was performed at request of physician and pathologic result was fibroadenoma, which was considered as concordant benign. B. At 6-month follow-up, mass was found to have grown along with development of calcifications and cystic change. C. Final pathological diagnosis of surgically excised lesion was malignant phyllodes tumor. Hypercellular stromal cells with atypia and mitosis (arrow) are noted (Hematoxylin & Eosin staining; original magnification, × 100).
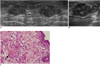
Fig. 5
52-year-old woman with palpable mass in her right breast.
A. On mediolateral oblique view of mammography, there is architectural distortion (arrow) in right breast. B. On sonography (left: transverse view, right: longitudinal view), irregular spiculated hypoechoic mass is seen and classified as BI-RADS category 4c. Result of sonography-guided 14-gauge core needle biopsy indicates stromal fibrosis, which was considered as discordant benign. C. Surgically excised specimen reveals widespread dense stromal fibrosis without ductal cell hyperplasia (arrow). Final diagnosis was stromal fibrosis (Hematoxylin & Eosin staining; original magnification, × 100).
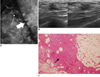
Fig. 6
65-year-old woman with mass in her right breast.
A. On mammography (left: mediolateral oblique view, right: craniocaudal view), indistinct mass (arrows) is present in right breast. B. On sonography (left: transverse view, right: longitudinal view), oval hypoechoic mass with angular or ill-defined margin is seen and classified as BI-RADS category 4c. After performing sonography-guided 14-gauge core needle biopsy, biopsy result was fibrocystic change, which is considered as discordant benign. C. Surgical specimen shows lesion consisting of extensive ductal carcinoma in situ with lobular cancerization. Relatively monotonous cells with low-grade nuclei and abundant, eosinophilic cytoplasm in form of cribriform pattern (arrow) are noted (Hematoxylin & Eosin staining; original magnification, × 200).
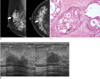
Fig. 7
36-year-old woman with palpable mass in her right breast.
A. On sonography (left: transverse view, right: longitudinal view), round microlobulated hypoechoic mass with microcalcifications and nonparallel orientation (arrowheads) is seen and classified as BI-RADS category 4c. Results of sonography-guided 14-gauge core needle biopsy indicate presence of stromal sclerosis, which is considered as discordant benign. B. Final diagnosis after surgical excision was invasive ductal carcinoma. Photomicrograph of microscopic specimen after surgical excision shows carcinoma within sclerotic stroma (Hematoxylin & Eosin staining; original magnification, × 40).

Fig. 8
40-year-old woman with palpable mass in her left breast.
A. On sonography (left: transverse view, right: longitudinal view), oval microlobulated hypoechoic mass with echogenic halo and nonparallel orientation is seen and classified as BI-RADS category 4b. Result of sonography-guided 14-gauge core needle biopsy indicates fibroadenoma, which is considered as discordant benign. B. Surgically excised specimen reveals well circumscribed, lobulated mass with tumoral florid adenosis, consistent with adenosis tumor (Hematoxylin & Eosin staining; original magnification, × 100).

Fig. 9
49-year-old woman with mass in her left breast.
A. On sonography (left: transverse view, right: longitudinal view), oval ill-defined hypoechoic mass (arrowheads) is seen and classified as BI-RADS category 4a. Results of sonography-guided 14-gauge core needle biopsy indicate presence of atypical intraductal papilloma and atypical ductal hyperplasia, which is considered as borderline or high-risk. B, C. At photomicrograph of microscopic specimen after surgical excision (B: Hematoxylin & Eosin staining; original magnification, × 10), ductal carcinoma in situ (upper and lower left; arrowheads) and atypical intraductal papilloma (upper right; arrow) are shown. At high-power field (C: Hematoxylin & Eosin staining; original magnification, × 100), ductal carcinoma in situ with low grade, cribriform pattern (left), as well as an atypical papilloma with central intraductal papilloma pattern and peripheral atypical ductal hyperplasia pattern in largest duct (right) are shown.
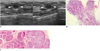
Fig. 10
49-year-old woman with palpable mass in her left breast.
A. On mediolateral oblique view of mammography, oval obscured mass (arrow) is shown in left breast. B. On sonography (left: transverse view, right: longitudinal view), oval circumscribed hypoechoic mass is seen and classified as BI-RADS category 3. Results of sonography-guided 14-gauge core needle biopsy performed by request of physician indicate presence of benign phyllodes tumor, which is considered as borderline or high-risk. C. Photomicrograph of microscopic specimen after surgical excision shows relatively ill-defined border, elongated epithelial-lined clefts, and mild increase in stromal cellularity with periductal stromal accentuation (arrowheads), consistent with benign phyllodes tumor (Hematoxylin & Eosin staining; original magnification, × 100).
References
1. Bassett LW, Mahoney MC, Apple SK. Interventional breast imaging: current procedures and assessing for concordance with pathology. Radiol Clin North Am. 2007. 45:881–894.
2. Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics. 2007. 27:79–94.
3. Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000. 89:2538–2546.
4. Parikh J, Tickman R. Image-guided tissue sampling: where radiology meets pathology. Breast J. 2005. 11:403–409.
5. Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002. 40:483–500.
6. Comstock CE. Feig SA, editor. US-guided interventional procedures. 2005 Syllabus: categorical course in diagnostic radiology-breast imaging. 2005. Oak Brook, IL: Radiological Society of North America;155–168.
7. Whitman GJ, Erguvan-Dogan B, Yang WT, Wilson J, Patel P, Krishnamurthy S. Ultrasound-guided breast biopsies. Ultrasound Clin. 2006. 1:603–615.
8. Starvros AT. McAllister L, Donnellan K, Martin SP, Rothschild R, editors. False-negative and false-positive examinations. Breast ultrasound. 2004. Philadelphia, PA: Lippincott Williams & Wilkins;947–978.
9. Kim MJ, Kim EK, Lee JY, Youk JH, Park BW, Kim SI, et al. Breast lesions with imaging-histologic discordance during US-guided 14G automated core biopsy: can the directional vacuum-assisted removal replace the surgical excision? Initial findings. Eur Radiol. 2007. 17:2376–2383.
10. Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol. 2008. 190:1209–1215.
11. Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006. 239:385–391.
12. Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management--follow-up and outcome. Radiology. 2008. 248:773–781.
13. Lee HJ, Kim EK, Kim MJ, Youk JH, Lee JY, Kang DR, et al. Observer variability of Breast Imaging Reporting and Data System (BI-RADS) for breast ultrasound. Eur J Radiol. 2008. 65:293–298.
14. American College of Radiology. American College of Radiology. Breast imaging reporting and data system-mammography. Breast imaging reporting and data system. 2003. 4th ed. Reston, VA: American College of Radiology.
15. Bent CK, Bassett LW, D'Orsi CJ, Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR Am J Roentgenol. 2010. 194:1378–1383.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download