Abstract
A congenital intrahepatic portosystemic shunt is a rare anomaly; but, the number of diagnosed cases has increased with advanced imaging tools. Symptomatic portosystemic shunts, especially those that include hyperammonemia, should be treated; and various endovascular treatment methods other than surgery have been reported. Hepatic masses with either an intra- or extrahepatic shunt also have been reported, and the mass is another reason for treatment. Authors report a case of a congenital intrahepatic portosystemic shunt with a hepatic mass that was successfully treated using a percutaneous endovascular approach with vascular plugs. By the time the first short-term follow-up was conducted, the hepatic mass had disappeared.
Acongenital intrahepatic portosystemic venous shunt (IPSVS) is a rare anomaly that manifests with symptoms of hepatic encephalopathy or hypoglycemia (1). Sometimes, these findings are found incidentally (2); however, when a portosystemic shunt carries an increased risk of hepatic encephalopathy or is associated with the development of liver tumors, it requires treatment (3). Treatment modalities vary and include dietary control, surgery and, recently, endovascular methods (4-7).
In this report, we present an 8-year-old male pediatric patient who had congenital IPSVS with a hepatic mass and hyperammonemia. He underwent successful treatment by percutaneous embolization with vascular plugs, and his hepatic mass also disappeared.
An 8-year-old boy was referred to our hospital due to abdominal pain. He had a history of neonatal hepatitis and had been diagnosed with congenital IPSVS six years before. The shunt was not corrected at that time because his parents were concerned about the possible complications associated with the therapeutic procedure. Currently, the boy is under medication for attention-deficit hyperactivity disorder. Laboratory values have shown a prolonged coagulation profile and slightly elevated ammonia level: prothrombin time, 20.1 sec; INR (international normalized ratio), 2.02; and ammonia, 55 Umol/L. Conservative management relieved his abdominal pain.
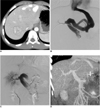
Abdominal ultrasonogram (US) demonstrated anechoic tubular structures connecting the left portal vein and the dilated left hepatic vein with a small-sized right portal vein. Color Doppler imaging revealed high-velocity, continuous waveform through the shunt; which are consistent with IPSVS. Enhanced computed tomography (CT) confirmed the sonographic findings. Three, variably-sized tubular vessels connected the left portal vein and the left hepatic vein, forming a loop-like appearance. In the liver's right lobe, we found a 3.5-cm sized mass, which showed heterogeneous nodular enhancement on the arterial phase and a mostly prolonged enhancement on five-minute delayed images (Fig. 1A). This mass was not found on the imaging study taken six years before. We thought it was possibly a benign tumor such as hemangioendothelioma or focal nodular hyperplasia (FNH), but we could not exclude a malignancy.
The patient's parents didn't want their child to undergo an invasive procedure for the IPSVS; thus, the pediatrician decided to conduct a close follow-up. At six-month follow-up, the boy's blood ammonia level had risen to 94 Umol/L, which is four times the normal range. A follow-up CT scan showed no significant change in the IPSVS and hepatic mass. Shunt ratio was calculated through the Doppler US (8), by dividing the blood flow volume at the shunt orifice (814 cc/min) by the total portal blood flow volume (882 cc/min). The shunt ratio was 92%, so we thought that the patient's condition could progress to hepatic encephalopathy. Therefore, we decided to close the shunt via endovascular treatment.
Under general anesthesia, an US-guided transhepatic right portal vein puncture was performed using a 22G needle, followed by the insertion of a 7 Fr, 24 cm sheath (Super Arrow-Flex: Arrow, PA). A direct portogram showed that the dilated left portal vein was connected directly with the dilated left hepatic vein by three variable tubular shunt channels (Fig. 1B). The right portal vein was very small compared to the left; hence, we chose to treat the boy with an Amplatzer vascular plug (AVP; AGA Medical, MN) because of the shunt's large size. A 20-mm AVP was deployed into the distal part of the largest shunt channel and two 12 mm AVPs were placed on the other smaller shunt channels. A post-embolization portography identified the absence of blood flow through the shunts and increased flow in the right portal vein (Fig. 1C). As we withdrew the sheath, we injected thick gelfoam slurry through the sheath's tract to avoid intra-abdominal bleeding. However, the patient's blood pressure suddenly dropped to 60/30 mmHg during the compression of the puncture site. US showed bleeding from the sheath's tract. We performed a vigorous resuscitation and US-guided compression of the bleeding focus. He experienced transient hypovolemic shock, but improved within 24 hours and recovered fully without further complication.
Six days after the procedure, the patient's serum ammonia level had decreased to within the normal range (25 Umol/L), and was discharged nine days post-embolization. An abdominal US performed two weeks later, showed occluded shunts and a slight decrease in the size of the hepatic mass. The right portal vein's blood flow had increased markedly with decreased main portal flow and minimally increased spleen size. A follow-up CT scan a month later showed complete embolization of the IPSVS along with improved portal flow to the right lobe (Fig. 1D), and nearly complete disappearance of the hepatic mass. Further, the patient was doing well at four months after embolization.
A congenital IPSVS is rare anomaly that results from abnormal embryologic development of the vitelline vein, or a variety of persistent patent ductus venosus (3, 6).
In patients with congenital IPSVS, some close spontaneously or can be asymptomatic for many years (3). Early diagnosis is important, even though patients can be asymptomatic, because the shunt's persistent patency causes hyperammonemia and increases the risk of hepatic encephalopathy (1, 9, 10).
Intrahepatic portosystemic venous shunt must be treated in patients with hepatic encephalopathy. In asymptomatic cases, the shunt ratio determines the treatment. The risk of encephalopathy increases with increasing shunt ratios. If the shunt ratio exceeds 60%, treatment is needed, even without hepatic encephalopathy (11). Our patient's shunt ratio was extremely high and his blood ammonia level was four times the normal range; thus, we decided on a therapeutic intervention.
Aside from dietary control, surgical operations such as shunt ligation and hepatic resection were performed in the past years (1, 4). Recently, however, less invasive percutaneous intervention has been widely adopted. Previous reports describe several percutaneous techniques for treating these shunts using various embolic agents and variable approach routes (5, 6, 12). However, we believe that our patient's shunts were too large to embolize with coils or other embolic agents, because of the potential complications of migration. Occlusion of the shunt risks causing portal hypertension (13). Our patient displayed mild splenomegaly; however, the size of the spleen did not differ significantly between the imaging studies performed two and four weeks after embolization. Considering the right portal vein's growth, we don't expect portal vein hypertension to progress; however, the boy will need additional close follow-up.
To our knowledge, no reported cases of congenital IPSVS have been treated by AVP, which can be used to occlude large, high-flow vessels. Recent reports do exist about the use of this device, other than the first introduced era (14, 15). Unlike embolization coils, an AVP can be positioned accurately, and migration of the AVP is uncommon. In the present case, we successfully occluded the large shunt using AVPs with clinical success and without AVP-related complications.
The bleeding episode, following shunt embolization in our patient, occurred through the sheath tract. A shunt occlusion must lead to an increase in blood flow and pressure in the right portal vein, and our method of tract embolization was insufficient for hemostasis. Transparenchymal tract embolization with different embolic agents including coils, gelfoam, and glue have been reported in cases of transhepatic portal vein approach or biopsy (16-19). An appropriate method must be chosen by considering the hemodynamic change after the procedure. Moreover, the confirmation of hemostasis is important.
Like our case, nodular liver lesions associated with congenital portosystemic shunts are not a rare condition (4, 20). Most of these lesions such as FNH or nodular regenerative hyperplasia, are benign (4). Regenerative liver lesions have been attributed to the diminution of portal flow and compensatory increase in hepatic arterial flow. The increased levels of hepatic growth factors through systemic circulation are also regarded as causative (4). Because the regenerative nodules are well described in congenital portosystemic shunts; and, because of the bleeding risk associated with a biopsy due to the hypervascular nature of CT scans, we decided to conduct a follow-up evaluation of the patient's mass lesion (21). Our patient's mass had been dramatically reduced after just a short follow-up period, which led us to conclude that the mass was most likely benign because of the increased portal flow to liver's right lobe, as described earlier (4).
In conclusion, IPSVS occlusion with an AVP is safe and effective, and shunt occlusion is a beneficial treatment method for regenerative liver lesions, as well as decreasing the risk of hepatic encephalopathy.
Figures and Tables
Fig. 1
Congenital intrahepatic portosystemic venous shunt and liver mass in child patient.
A. Contrast-enhanced multidetector CT scan performed before embolization revealed 3.5 cm mass with heterogeneous enhancement in liver's right lobe.
B. Right anterior oblique digital subtraction direct portogram demonstrated direct connection between dilated left portal vein and left hepatic vein by three variable shunt channels. Right portal vein was very small, compared to left portal vein.
C. Right anterior oblique digital subtraction direct portogram immediately after embolization showed successful occlusion of intrahepatic portosystemic shunt and improvement of flow through right portal vein.
D. Oblique coronal reformatted CT scan image one month after procedure showed markedly increased size of right portal veins, compared to B.
References
1. Chagnon SF, Vallee CA, Barge J, Chevalier LJ, Le Gal J, Blery MV. Aneurysmal portahepatic venous fistula: report of two cases. Radiology. 1986. 159:693–695.
2. Singh K, Kapoor A, Kapoor A, Gupta K, Mahajan G. Congenital intrahepatic portosystemic shunt--an incidental rare anomaly. Indian J Pediatr. 2006. 73:1122–1123.
3. Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008. 21:147–157.
4. Kanamori Y, Hashizume K, Kitano Y, Sugiyama M, Motoi T, Tange T. Congenital extrahepatic portocaval shunt (Abernethy type 2), huge liver mass, and patent ductus arteriosus--a case report of its rare clinical presentation in a young girl. J Pediatr Surg. 2003. 38:E15.
5. Ikeda S, Sera Y, Yoshida M, Izaki T, Uchino S, Endo F, et al. Successful coil embolization in an infant with congenital intrahepatic portosystemic shunts. J Pediatr Surg. 1999. 34:1031–1032.
6. Yoon HK, Choo SW, Do YS, Choo IW, Han BK. Congenital intrahepatic portosystemic shunts in the neonate: coil embolization via the umbilical vein. J Vasc Interv Radiol. 1998. 9:509–511.
7. Fanelli F, Marcelli G, Bezzi M, Maria Salvatori F, Rossi M, Rossi P, et al. Intrahepatic aneurysmal portohepatic venous shunt: embolization with a tissue adhesive solution. J Endovasc Ther. 2003. 10:147–153.
8. Kudo M, Tomita S, Tochio H, Minowa K, Todo A. Intrahepatic portosystemic venous shunt: diagnosis by color Doppler imaging. Am J Gastroenterol. 1993. 88:723–729.
9. Park JH, Cha SH, Han JK, Han MC. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990. 155:527–528.
10. Kerlan RK Jr, Sollenberger RD, Palubinskas AJ, Raskin NH, Callen PW, Ehrenfeld WK. Portal-systemic encephalopathy due to a congenital portocaval shunt. AJR Am J Roentgenol. 1982. 139:1013–1015.
11. Uchino T, Matsuda I, Endo F. The long-term prognosis of congenital portosystemic venous shunt. J Pediatr. 1999. 135:254–256.
12. Kim IO, Cheon JE, Kim WS, Chung JW, Yeon KM, Yoo SJ, et al. Congenital intrahepatic portohepatic venous shunt: treatment with coil embolisation. Pediatr Radiol. 2000. 30:336–338.
13. Okada Y, Endo T, Kusano S, Yoshida M. Multiple intrahepatic portohepatic venous shunts: treatment with steel-coil embolization. AJR Am J Roentgenol. 1991. 157:971–973.
14. Kessler J, Trerotola SO. Use of the Amplatzer Vascular Plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Interv Radiol. 2006. 17:135–140.
15. Pattynama PM, Wils A, van der Linden E, van Dijk LC. Embolization with the Amplatzer Vascular Plug in TIPS patients. Cardiovasc Intervent Radiol. 2007. 30:1218–1221.
16. Crummy AB, McDermott JC, Wojtowycz M. A technique for embolization of biopsy tracts. AJR Am J Roentgenol. 1989. 153:67–68.
17. Lyon SM, Terhaar O, Given MF, O'Dwyer HM, McGrath FP, Lee MJ. Percutaneous embolization of transhepatic tracks for biliary intervention. Cardiovasc Intervent Radiol. 2006. 29:1011–1014.
18. Litvin S, Atar E, Knizhnik M, Bruckheimer E, Belenky A. Stent graft closure of a high flow splenorenal shunt after liver transplantation. Diagn Interv Radiol. 2009. [Epub ahead of print].
19. Yankes JR, Uglietta JP, Grant J, Braun SD. Percutaneous transhepatic recanalization and thrombolysis of the superior mesenteric vein. AJR Am J Roentgenol. 1988. 151:289–290.
20. Sawyer B, Dow C, Frank J, Lau E. Congenital portocaval shunt-associated liver lesions in a patient with cancer. ANZ J Surg. 2008. 78:613–614.
21. Kandpal H, Sharma R, Arora NK, Gupta SD. Congenital extrahepatic portosystemic venous shunt: imaging features. Singapore Med J. 2007. 48:E258–E261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download