Abstract
We report a case of a pulmonary venous malformation in a 4-year-old boy who presented with recurrent pneumonia. A radiograph revealed a right infrahilar mass and a hyperlucent right lung. Computed tomography (CT) demonstrated a mass containing intensely enhancing areas and multiple phleboliths located in the right lower lobe and encasing the right bronchus and right inferior pulmonary vein. Magnetic resonance imaging (MRI) precisely revealed the mass demarcation. A right lower lobectomy was performed and a pathological examination confirmed the diagnosis of a venous malformation. To the best of our knowledge, a venous malformation in pulmonary tissue has not been reported in the English literature. Herein, we report a case of a pulmonary venous malformation, with the radiograph, CT, MRI, and blood pool scan findings, along with its pathologic correlation.
Avenous malformation is part of the spectrum of vascular malformations occasionally found in children. Venous malformations can be found anywhere in the body. However, they are most commonly found in the soft tissues of the head and neck (40%), extremities (40%) and trunk (20%) (1). To the best of our knowledge, a pulmonary venous malformation has not been previously reported in the English literature. Here, we report a case of pulmonary venous malformation in a 4-year-old boy.
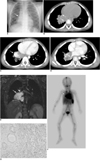
A 4-year-old boy visited a local hospital due to repeated episodes of pneumonia since the age of three months. A chest radiograph and CT revealed an abnormal mass-like lesion in the right lower pulmonary lobe. An attempted total excision was unsuccessful, and only a biopsy was performed in the local hospital. The precise pathologic report of the outside hospital was not available except for information of a kind of vascular mass. Because the boy had long-standing and recurrent symptoms, he was referred to our vascular center for further evaluation and treatment. A plain radiograph showed increased right infrahilar opacity overlapping the cardiac shadow. The right lung was more lucent and smaller than the left one, suggesting an airway problem (Fig. 1A). A precontrast CT scan revealed a soft tissue mass containing multiple and well-defined small calcific foci in the right lower lobe of the lung, which were suggested to be phleboliths (Fig. 1B). After injecting the contrast material, the anterolateral portion of the mass was intensely enhanced without enhancement of the surrounding region (Fig. 1C). The lesion was encasing the right bronchus intermedius and the right lower lobar bronchus, resulting in decreased caliber. The right inferior pulmonary vein was also narrowed by the encasing mass (Fig. 1D). The mass abutted the posterior margin of the heart and superior pulmonary vein. Before surgery, an MRI was performed in our hospital three months after the CT scan performed at the outside hospital to further evaluate the mass demarcation with the surrounding tissues; especially in the airway and large vessels. The lesion was found to be isointense with the muscles on T1-weighted images and hyperintense on the T2-weighted images (Fig. 1E). There were no signal differences between the enhancing and the non-enhancing part on a contrast enhanced CT on MRI. Because the intravenous administration of MRI contrast agent was not performed during MRI, the comparison of the enhancing pattern between the CT and MRI was not possible. Linear or dot-like signal voids within the mass suggested vessels and phleboliths. This mass abutted the right pulmonary artery and the pericardium, but there was no evidence of invasion into the myocardium. A whole body blood pool scintigraphy (WBBPS) was performed one hour after the injection of Tc-99m RBC. The results demonstrated dense abnormal blood pooling in the right infrahilar area similar to that of the heart, and small multifocal blood poolings in the right upper and the left lower extremities (Fig. 1F). According to the imaging findings and the pathologic report of the outside hospital, a vascular malformation was the most probable preoperative diagnosis.
Because the lesion caused clinical problems, including frequent infection and compression of the airway, a right lower lobectomy was performed. A soft mass abutting the right pulmonary artery at the interlobar fissure was found at surgery. The mass adhered to the pericardium near the right inferior pulmonary vein. The resected specimen was identified as a poorly defined hyperemic mass measuring 5 × 5 × 3 cm at the right lower lobe of the lung. The mass was composed of thin-walled, dilated, sponge-like abnormal vascular channels of variable size and thickness. Because there was no elastic tissue in the wall of the vessels, a venous malformation was diagnosed (Fig. 1G). There were no pathologic differences between the enhancing and the non-enhancing parts on a contrast-enhanced CT.
The patient has been followed uneventfully for 26 months after surgery. Follow-up chest radiographs and chest CT scan were taken every 12 months following surgery and have not revealed any evidence of a recurrent lesion. However, the vascular lesions in the extremities slightly increased in size on a follow-up WBBPS, probably combined with somatic growth.
The final classification of vascular anomalies was updated by of the International Society for the Study of Vascular Anomalies (ISSVA) in 1996. Vascular anomalies are classified into two major categories: hemangiomas and vascular malformations. Hemangiomas are benign tumors of infancy characterized by a rapid, proliferative phase lasting 8 to 10 months, followed by slow, spontaneous involution over a period of several years. Hemangiomas are composed of proliferating endothelial cells. In contrast, vascular malformations are composed of dysplastic vessels without endothelial proliferation. They are further categorized according to the type of vessel involved (capillary, venous, arterial, lymphatic, or combinations of these) and according to their hemodynamic features (high flow versus low flow) (1). Two-thirds of congenital vascular malformations are venous; whereas, a quarter of these lesions are completely or partly of lymphatic origin, and are named low- or slow-flow malformations. The remaining vascular malformations are considered to be high-flow malformations (2).
Venous malformations occur anywhere in the body, but are most commonly observed in the head and neck (40%), extremities (40%), and trunk (20%) (1). Most venous malformations are solitary, but can sometimes be multiple. In this case, multiple venous malformations in both extremities, aside from the pulmonary lesion were also suggested in the WBBPS. Osler-Wever-Rendu syndrome can also show multiple vascular lesions. However, this patient did not have recurrent epistaxis or such a family history. Also the lung lesion was not an arteriovenous malformation, but a venous malformation. Several syndromes are comprised of venous malformations in whole or in combination with other malformations (1), but this patient did not have any feature to suggest a type of syndrome except for multiple venous malformations.
Venous malformations usually vary between relatively small, well-circumscribed, superficial lesions, to large, infiltrative lesions crossing multiple soft tissue planes involving subcutaneous fat, bone, neurovascular bundles, or even viscera. They are bluish in color and do not show a local increase in temperature. Venous malformations typically increase in size during the Valsalva maneuver and are easily compressible and non-pulsatile. However, deeper lesions are impossible to evaluate properly on clinical criteria alone, and are frequently more extensive than initially expected (1, 2). Venous malformations are often asymptomatic. However, symptoms may appear in cases of thrombophlebitis, or with muscular or articular involvement. In this case, the lesion caused bronchial narrowing and consequent recurrent pneumonia.
The basic unit of the developing lung are the conducting airway, the alveolus, and the preacinar blood vessel (3). A congenital mass of the lung is thought to occur when an error occurs in the developing processes of one or more of the basic structural components. Those congenital malformations are not isolated abnormalities of lung formation, but a spectrum or continuum of interrelated abnormalities (4). One end of this spectrum is a lesion with normal vasculature, but abnormal pulmonary parenchyma such as congenital lobar overinflation. The other end of the spectrum is the pulmonary vascular malformation with normal parenchyma (5). A pulmonary venous malformation may be a kind of malformation in this spectrum without parenchymal malformation. There are few reported cases of pulmonary hemangiomas in the English literature (6, 7). However, a pulmonary venous malformation has not been previously reported in the English literature.
A variety of radiological modalities, including CT, MRI, venography, and Doppler ultrasonography are used for the evaluation and diagnosis of venous malformations. The CT appearance of a venous malformation is a hypoattenuating or heterogeneous lesion that enhances slowly and peripherally after a bolus injection of contrast material. Typical phleboliths are easily seen on CT. Further, an MRI provides more accuracy for the evaluation of the extent of lesions and their relationship to other structures. A venous malformation most commonly has intermediate signal intensity on T1- and high signal intensity on T2-weighted images (1).
The unique location of the venous malformation, in this case, caused airway obstruction and repeated infection, ultimately leading to the decision to perform a surgical resection. This case study shows that a venous malformation can exist in the lung parenchyma and cause symptoms. Therefore, radiologists should be aware of this possibility and the imaging findings of a venous malformation.
Figures and Tables
Fig. 1
4-year-old boy with repeated episodes of pneumonia.
A. Chest radiograph shows right lower lobe mass (arrow), unilateral hyperlucency, and decreased volume of right lung.
B. Precontrast CT scan shows multiple phleboliths within mass (arrows).
C. After injection of contrast material, anterior portion of mass was intensely enhanced (arrow).
D. Mass caused luminal narrowing of right lower lobar bronchus (arrowhead) and right inferior pulmonary vein (arrow).
E. T2-weighted coronal images show high-intensity mass encasing pulmonary artery (large arrow), bronchus (small arrow), and pulmonary vein (arrowhead).
F. Whole body blood pool scintigraphy demonstrates intense blood pooling at right infrahilar region (arrow), similar to that of heart and multifocal faint blood poolings in right upper and left lower extremities (arrowheads).
G. Microscopic image demonstrates typical erythrocyte-filled vascular spaces lined with flattened mature nonhypercellular endothelium without elastic tissue in wall of vessels (Elastic Stain, ×10).
References
1. Legehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008. 46:545–597.
2. Abernethy LJ. Classification and imaging of vascular malformations in children. Eur Radiol. 2003. 13:2483–2497.
3. Panicek DM, Heitzman ER, Randall PA, Groskin SA, Chew FS, Lane EJ Jr, et al. The continuum of pulmonary developmental anomalies. Radiographics. 1987. 7:747–772.
4. Puig S, Casati B, Staudenherz A, Paya K. Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol. 2005. 53:35–45.
5. Reid LM. Lung growth in health and disease. Br J Dis Chest. 1984. 78:113–134.
6. Galliani CA, Beatty JF, Grosfeld JL. Cavernous hemangioma of the lung in an infant. Pediatr Pathol. 1992. 12:105–111.
7. Wodenhouse GE. Hemangioma of the lung: a review of four cases, including two not previously reported, one of which was complicated by brain abscess due to H. influenzae. J Thorac Surg. 1948. 17:408–415.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download