Abstract
Objective
The objective of this study was to evaluate the safety and therapeutic efficacy of percutaneous radiofrequency (RF) ablation for the treatment of hepatocellular carcinomas (HCCs) adjacent to the gallbladder with the use of internally cooled electrodes.
Materials and Methods
We retrospectively assessed 45 patients with 46 HCCs (mean size, 2.2 cm) adjacent to the gallbladder (≤1.0 cm) treated with RF ablation using an internally cooled electrode system. An electrode was inserted into the tumor either parallel (n = 38) or perpendicular (n = 8) to the gallbladder wall. The safety and therapeutic efficacy of the procedures were assessed with clinical and imaging follow-up examinations. Follow-up with the use of CT ranged from four to 45 months (mean, 19 months). The association between variables (electrode direction, electrode type, tumor size, tumor location, lobar location) and the presence of a residual tumor or local tumor progression was also analyzed.
Results
There were no major complications and minor complications were noted in three patients (7%) including one case of vasovagal syncope and two cases of bilomas. Wall thickening of the gallbladder adjacent to the RF ablation zone was noted in 14 patients (41%) as determined on immediate follow-up CT imaging. Wall thickening showed complete disappearance on subsequent follow-up CT imaging. The primary technique effectiveness rate was 96% (44/46) based on one-month follow-up CT imaging. Local tumor progression was noted in six (14%) of 44 completely ablated tumors during the follow-up period. The direction of electrode insertion (perpendicular), tumor size (≥3 cm) and tumor location (a tumor that abutted the gallbladder) were associated with an increased risk of early incomplete treatment. No variable was significantly associated with local tumor progression.
Radiofrequency (RF) ablation has been regarded as a safe and effective technique for the treatment of hepatocellular carcinomas (HCCs) (1-5). In recent studies, for small HCCs, it has been reported that the survival rate after RF ablation was comparable to the survival rate after surgery (6, 7). With sufficient experience, RF ablation can be performed easily and safely. However, the procedure might be limited by intrahepatic or extrahepatic factors. The heat sink effect decreases the extent of coagulation necrosis in tumors that are close to large vessels (8). In addition, collateral thermal damage of adjacent extrahepatic organs can occur when treating subcapsular tumors. The gallbladder is at risk for potential thermal damage; complications such as perforation or cholecystitis can develop after RF ablation (9, 10).
Several studies that have reported on the safety and efficacy of RF ablation for hepatic tumors adjacent to the gallbladder are found in the medical literature (11-14). However, prior studies have used only multi-tined expandable electrodes (11, 12) or had a limited study population (13, 14). Therapeutic failure after RF ablation can appear as the presence of a residual unablated tumor or local tumor progression. However, to the best of our knowledge, no previous study has focused on the risk factors for therapeutic failure after RF ablation for an HCC adjacent to the gallbladder. The goal of this study was to evaluate the safety and therapeutic efficacy of percutaneous RF ablation using an internally cooled electrode system for the treatment of HCCs adjacent to the gallbladder and to assess the risk factors for therapeutic failure after the procedure.
From July 2000 to February 2006, 627 patients with nodular HCCs were treated using RF ablation with an internally cooled electrode system at our institution. We reviewed our institutional database for RF ablation cases and identified 45 patients who had 46 HCCs adjacent to the gallbladder. All but one patient had one tumor. Two HCCs that occurred at different times of the study period in one patient were ablated in two different sessions. The patient population included 33 men and 12 women (age range, 35-86 years; mean age, 62.9 years). Twenty-six patients were not candidates for surgical treatment or had unresectable lesions; 19 patients had resectable tumors but refused surgical treatment.
The maximum diameter of the tumors ranged from 0.7 cm to 4.3 cm (mean, 2.2 cm; standard deviation [SD], 0.76 cm) based on planning ultrasound. Twenty-one tumors (mean diameter, 2.2 cm; range, 1.0-4.3 cm) abutted the gallbladder and 25 tumors were located within 1 cm (range, 0.2-1.0 cm; mean distance, 0.6 cm) of the gallbladder. Fifteen tumors (mean diameter, 2.2 cm; range, 0.7-4.0 cm) were located within 0.5 cm of the gallbladder and 10 tumors (mean diameter, 2.1 cm; range, 1.1-3.5 cm) were located within 0.6-1.0 cm of the gallbladder. The distance between the tumor and the gallbladder was measured as the shortest distance on an axial image. If the tumor and gallbladder were not seen on the same image, the distance was calculated by multiplying the number of sections that were included between the two entities by the slice thickness. Thirty-two tumors were located in liver segment V, 13 tumors were located in liver segment IV, and one tumor was located in liver segment VIII.
Twelve patients had gallstones in the gallbladder. Ten patients had gallbladder wall edema related to cirrhosis as detected on CT scans obtained before RF ablation. These patients had neither right upper quadrant pain nor Murphy's sign. The criteria for RF ablation for an HCC at our hospital was the following. Criteria included the presence of a single nodular HCC that was less than 5 cm in the maximum diameter. Patients could have up to three multinodular HCCs, with each tumor measuring up to 3 cm in diameter. Patients presented with no portal venous thrombosis or an extrahepatic metastasis. Patients had Child-Pugh classification A or B liver cirrhosis. Patients had a prothrombin time ratio greater than 50% and a platelet count greater than 70,000/mm3. Institutional Review Board approval and patient written informed consent were obtained routinely prior to performing RF ablation therapy.
The diagnosis of HCC was based on the results of a percutaneous biopsy for five tumors and the characteristic enhancement pattern depicted on contrast-enhanced multiphase helical CT with or without elevated serum tumor markers (n = 11; α-fetoprotein level > 200 ng/mL [> 200 µg/L]) for the remaining 41 tumors. Of the 45 patients, 42 patients had liver cirrhosis due to hepatitis B (n = 30) or hepatitis C (n = 12) infection. The remaining three patients had chronic hepatitis C without liver cirrhosis. Among the 42 cirrhotic patients, 30 patients had Child-Pugh class A cirrhosis and 12 patients had Child-Pugh class B. Twenty patients had a history of previous treatment with transcatheter arterial chemoembolization (TACE) (n =13), RF ablation (n = 1) or both methods (n = 6). One patient had a history of undergoing a tumorectomy for HCC.
Between July 2000 to February 2006, the majority of percutaneous RF ablations at our hospital were performed using an internally cooled electrode system (Cool-tip RF ablation system; Valleylab, Boulder, CO). This device consists of a needle electrode with a tip internally cooled by chilled saline and a 200-watt generator. For this study, all RF ablation procedures were performed with the internally cooled electrode system under real-time sonographic guidance (HDI 5000 unit, ATL; Philips Medical Systems, Bothell, WA; Sequoia 512 unit, Siemens Medical Solutions, Erlangen, Germany). All patients who underwent percutaneous RF ablation received a 50 mg intramuscular injection of pethidine hydrochloride (Pethidine; Samsung Pharmaceutical, Seoul, Korea) before the procedure and local anesthesia with the use of lidocaine (Kwang Myung Pharmaceutical, Seoul, Korea). In addition, additional pethidine hydrochloride (50 mg) was administered to patients who complained of intolerable pain during the ablation procedure. During the procedure, vital signs were monitored continually. Power was applied from 50 watts and was gradually increased to the maximum level for one minute if the patient could tolerate the procedure with stable vital signs. The mean time for application of RF was 12 minutes (range, 6-15 minutes). In three cases with large tumors, two overlapping ablations were performed to cover the entire tumor. We used a 2-cm single straight electrode in 10 cases, a 3-cm single straight electrode in 31 cases and a clustered electrode for five cases (size range of index tumors, 3.0-4.0 cm; mean size, 3.5 cm) according to the size and location of the tumors. In general, ablation of an additional 0.5-1 cm of the normal liver parenchyma around the tumor was necessary to minimize local tumor progression. However, it was difficult to obtain a sufficient safety margin surrounding the tumor close to the gallbladder, especially in areas that abutted the gallbladder. We classified the direction of electrode insertion into two categories, parallel or perpendicular, based on whether the tip of electrode was inserted towards the gallbladder wall (perpendicular) or not (parallel) (Fig. 1). The direction of electrode insertion was chosen according to the feasibility of electrode path. Eight tumors were treated with electrodes inserted perpendicularly and 38 tumors were treated with electrodes inserted parallel. When inserting a needle electrode into the tumor parallel to the gallbladder wall, maintenance of a distance of at least 1.0 cm between the center of the active electrode tip and the gallbladder wall was attempted. In cases where the needle electrode was placed perpendicular to the gallbladder wall, a distance of at least 0.5 cm was maintained between the electrode tip and the gallbladder wall. The safety distances mentioned above were based on the results reported by Ng et al. (15). If a sufficient safety distance from the gallbladder wall could not be obtained, especially in cases of small-sized tumors, we located the electrode off center in the tumor. Further details on the ablation procedures have been reported previously (16, 17).
After RF ablation, contrast-enhanced CT examinations were performed within two hours (n = 31) or contrast-enhanced sonography was performed one day after the procedure (n = 12) or both types of examinations were performed (n = 3) to assess the completeness of the ablation and to detect the presence of immediate complications. Contrast-enhanced sonography was performed after injection of a microbubble contrast agent (Levovist; Schering, Berlin, Germany) in 15 patients according to the preference of the radiologists. Contrast-enhanced CT examinations were performed with helical scanners (HiSpeed Advantage or LightSpeed QX/I, GE Healthcare, Milwaukee, WI).
All patients underwent follow-up with contrast-enhanced three-phase CT at one-month and then every two to four months after the ablation. Follow-up with CT ranged from four to 45 months (mean follow-up, 19 months). Eight patients died within one year (mean time of death, six months) due to disease progression, regardless of the procedure, and seven patients underwent follow-up for less than 12 months; two patients with residual unablated tumors received additional TACE and five patients developed local tumor progression within 12 months after RF ablation. The 31 remaining patients underwent follow-up for at least 12 months (≥12 months, n = 18; ≥24 months, n = 10; 3≥6 months, n = 3). Immediate complications were assessed by the use of contrast-enhanced sonography or an immediate follow-up CT scan. However, evaluation of therapeutic efficacy of RF ablation was performed at the one-month follow-up with a CT scan. If any residual unablated tumor or local tumor progression was noted on follow-up CT, additional therapeutic procedures such as RF ablation or TACE were attempted.
Three abdominal radiologists retrospectively reviewed the CT scans to evaluate the complications and therapeutic efficacy. The radiologists arrived at a consensus interpretation of the imaging findings. On follow-up imaging, the presence of gallbladder wall thickening and abnormal fluid collection around the gallbladder was evaluated. CT scans were performed immediately for the evaluation of gallbladder wall thickening. A total of 34 patients including six patients who had gallbladder wall edema related to cirrhosis were evaluated for gallbladder wall thickening. Thickening of the gallbladder wall was defined as a thickness greater than 2 mm (13). In cases of gallbladder wall edema related to cirrhosis, we evaluated whether mucosal enhancement was present in the wall. Therapeutic efficacy was assessed by evaluating patients for the presence of a residual unablated tumor, primary technique effectiveness and local tumor progression. A residual unablated tumor was defined as the presence of any remaining enhancing foci in the ablation zone as depicted on either contrast-enhanced sonography or early follow-up CT immediately or at one month after the procedure (18, 19). The primary technique effectiveness rate was defined as the percentage of tumors that were completely ablated after the initial procedure, as evidenced on one-month follow-up CT (19). Local tumor progression was defined as newly appearing enhancing tumors at or contiguous to the ablation zone as depicted on follow-up CT after one month (19). The definitions used for major and minor complications followed Society of Interventional Radiology recommendations (19). Patient medical records were reviewed to evaluate symptoms during and after ablation. In all patients, whether pain was experienced during or after the procedures was recorded in the medical records, including the radiological records. In addition, we evaluated if pain was tolerated without the administration of analgesics or was severe and required the administration of analgesics.
Associations between gallbladder wall thickening and other variables including tumor location, post-procedural pain and electrode direction were evaluated using the χ2 test or Fisher's exact test. The univariate association between variables (for the direction of electrode insertion, type of electrode, tumor size, tumor location and lobar location) and the presence of a residual tumor or local tumor progression was assessed by the use of Fisher's exact test or the χ2 test for trend. P values of less than 0.05 were considered statistically significant. Data analyses were performed using SPSS for Windows (version 12.0; SPSS. Chicago, IL).
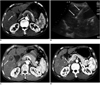
There were no treatment-related deaths in the patient population. In addition, there were no major complications such as cholecystitis or gallbladder perforation. Three minor complications (3 of 46, 7%) were noted: one case of vasovagal syncope and two cases of bilomas. In the patient where vasovagal syncope occurred, a single ablation was continued with the use of a lower power setting (5-25 W) for 15 minutes after recovery from syncope. However, in this patient, the presence of a residual unablated tumor was demonstrated on a contrast-enhanced CT scan obtained immediately after RF ablation (Fig. 2). Two patients with bilomas showed no progression as seen on follow-up CT and did not require any treatment.
During the procedure, eight patients (8 of 46, 17%) complained of severe pain and pain was controlled with an additional 50 mg IV of pethidine HCl. After the ablation, severe abdominal pain that required the administration of analgesics was noted in five patients (5 of 46, 11%). Eight patients (8 of 46, 17%) presented with low-grade fever (range, 37.2-38.0℃; mean, 37.5℃) after the procedure that resolved spontaneously after one to two days. Nausea occurred in three patients (3 of 46, 7%). Right side pleural effusion occurred in one patient as seen on an immediate follow-up CT scan and pleural effusion was improved as demonstrated on one-month follow-up CT.
Focal wall thickening of the gallbladder adjacent to the RF ablation zone was noted in 14 cases (14 of 34, 41%) as seen on the immediate follow-up CT. The mean wall thickness of the gallbladder in the 14 cases was 2.7 mm (range, 2.0-4.0 mm). Minimal wall thickening of the gallbladder (2.0-3.0 mm, n = 9) was seen as focal wall enhancement adjacent to the RF ablation zone. A more thickened wall (> 3.0 mm, n = 5) was seen as a hypodense outer layer, a similar appearance to subserosal edema. Three of six patients who had gallbladder wall edema related to liver cirrhosis prior to RF ablation had mild mucosal enhancement. Gallbladder wall thickening was noted in eight (8 of 14, 57%) of the 14 HCCs that abutted the gallbladder and in six cases (6 of 20, 30%) among the 20 HCCs that did not abut the gallbladder; this difference was not statistically significant (p = 0.2). Seven (7 of 14, 50%) of 14 patients with gallbladder wall thickening complained of upper abdominal pain and 13 patients (13 of 20, 65%) without gallbladder wall thickening complained of pain. Differences in post-procedural pain according to the presence or absence of gallbladder wall thickening were not statistically significant (p = 0.5). The proportion of gallbladder wall thickening in patients who underwent RF ablation with parallel direction procedures (11 of 27, 41%) were similar to the proportion of wall thickening in patients who underwent RF ablation with perpendicular direction procedures (3 of 7, 43%); there was no statistical significance according to the electrode direction (p = 1.0). In all cases, subsequent follow-up CT demonstrated the disappearance of gallbladder wall thickening (Figs. 3, 4). In addition, all patients with gallbladder wall thickening had a normal WBC count (less than 8,000 cells/mm3).
Of the 46 tumors, four (4 of 46, 9%) cases had residual tumors noted on immediate follow-up imaging (CT, n = 2; contrast-enhanced US, n = 1) and one-month follow-up CT (n = 1) (Table 3) (Fig. 2). All tumors abutted the gallbladder. The mean diameter of index tumors with a residual tumor was 3.3 cm. The electrode direction to the gallbladder wall was perpendicular in three cases and parallel in one case. The type of electrode used was a single straight electrode in all cases with a residual tumor. Based on the use of univariate analyses, the direction of electrode insertion (perpendicular), tumor size (≥3 cm) and tumor location (a tumor that abutted the gallbladder) were significantly associated with the risk of incomplete treatment (p < 0.05) (Table 1). Two residual unablated tumors were treated again with a second session of RF ablation on the next day and no residual unablated tumor was seen on one-month follow-up CT images. The remaining two tumors were treated with TACE. Thus, the primary technique effectiveness rate as depicted on one-month follow-up CT was 96% (44 of 46).
Of the 44 tumors that were successfully treated with no evidence of a residual unablated tumor on one-month follow-up CT, six tumors (6 of 44, 14%) showed local tumor progression as seen on follow-up CT performed at three months (n = 1), four months (n = 3), five months (n = 1) and 17 months (n = 1) (Table 4) (Fig. 5). The maximum diameter of the index tumors ranged from 0.7 cm to 3.6 cm (mean, 2.6 cm) as measured on sonography. The site of recurrence was the RF ablation zone near the gallbladder for three tumors and the opposite portions of the ablation zone away from the gallbladder for three tumors. No variable was significantly associated with local tumor progression based on the use of univariate analyses (p > 0.05) (Table 2). Of the six patients with local tumor progression, five patients were treated with TACE and one patient underwent liver transplantation. During the study period, newly developed HCCs at a site remote from the ablated tumor were noted in 19 of 43 patients (44%). Twelve patients died during the study period.
Although the use of RF ablation has increased, several technical limitations remain. Among the limitations, tumors near the hepatic hilum, large vessels, gastrointestinal tract, gallbladder, diaphragm and heart can be difficult to treat completely (11-14, 18). RF ablation for these tumors may result in incomplete necrosis of the index tumor or collateral thermal damage to adjacent organs. Therefore, special precautions are needed to treat tumors in these dangerous locations.
Although RF ablation of tumors adjacent to the gallbladder is always accompanied by the risk of gallbladder perforation or acute cholecystitis, recent clinical studies have shown that this procedure can be safely and effectively performed (11-14). Chopra et al. (13) first reported that percutaneous RF ablation was a feasible and safe procedure to perform for hepatic tumors located adjacent to the gallbladder. A few subsequent studies have yielded similar results for the effectiveness and safety of this procedure (11, 12, 14). However, the studies have adopted different types of electrodes and techniques; expandable electrodes were used in two studies (11, 12) and cool-tip electrodes were used in one study (14). In one study, an aseptic solution was injected into the gallbladder fossa (12).
The results of the current study support the findings of prior reports claiming that RF ablation for hepatic tumors adjacent to the gallbladder is a safe procedure. We found neither procedure-related death nor major complications that required treatment, consistent with previous investigations (11-14). Three minor complications in our study were noted. A vasovagal reaction occurred in one patient, and this eventually led to incomplete treatment. An RF study of 1,663 hepatic tumors in 1,139 patients performed in South Korea had two cases of vasovagal reaction (20). Although it is uncertain as to whether RF ablation for tumors adjacent to the gallbladder increases the risk for vasovagal reaction, stimulation of the gallbladder may cause gallbladder-cardiac syndrome (12). Therefore, continuous monitoring of vital signs during the procedure is mandatory.
Although there was no mortality or significant morbidity in our study, we found that RF ablation for an HCC near the gallbladder had a definite thermal effect on the gallbladder wall as seen on immediate follow-up imaging. Our findings support the results of a previous report (13) that found focal transient thermal injury of the gallbladder wall after RF ablation. These results suggest that RF ablation around the gallbladder causes reversible thermal injury of the gallbladder wall due to thermal conduction. However, severe complications such as overt cholecystitis or perforation were not encountered; these findings may be explained by the presence of bile in the gallbladder, which acts as a source of heat convection. In an experimental study using a pig model, thermal injury to the gallbladder wall after RF ablation has also been demonstrated (21). In addition, this study showed that RF ablation of hepatic tumors that abutted the gallbladder could cause perforation of the gallbladder unless a safe distance between the electrode and the gallbladder was obtained. This serious complication would present as a delayed process (up to seven days). Although perforation of the gallbladder did not occur in any of the 21 patients with tumors that abutted the gallbladder included in this study, we think that any patient undergoing RF ablation near the gallbladder without a sufficient safety distance should be observed carefully for at least seven days after the procedure.
The incidence of gallbladder wall thickening in other studies [3 of 53 cases, 6% (11); 3 of 49 cases, 6% (12); 6 of 23 sessions, 26% (13)] differed from the present study (14 of 34 cases, 41%). The size of the index tumor, the type of electrode used and the criteria for gallbladder wall thickening might be considered as causes of these differences. In this study, we found the presence of gallbladder wall thickening after RF ablation was not related to tumor location, the development of post-procedural pain and electrode direction. A previous experimental study showed that full-thickness thermal injury to the gallbladder was more frequent in cases with the use of parallel direction procedures (5 of 12, 42%) as compared to cases with the use of perpendicular direction procedures (2 of 11, 18%) (21). However, a relatively small number of cases with the perpendicular direction procedure were included in our study and RF ablation for a normal pig liver instead of a tumor model was performed in the previous experimental study. Therefore, a comparison between these clinical and experimental studies may be limited.
The primary technique effectiveness rate in the current study (96%) was comparable to previously reported studies using multi-tined expandable electrodes [91% (11) and 92% (12)]. The fact that the index tumor diameter in our study (mean diameter, 2.3 cm) was smaller than previously reported may have contributed to the slightly improved early therapeutic effectiveness in our study. In this study, the direction of electrode insertion (perpendicular), tumor size (≥3 cm), and tumor location (tumors that abutted the gallbladder) were risk factors of early incomplete treatment. In addition, there were no residual tumors for relatively large tumors (mean, 3.5 cm; n = 5) treated with a clustered electrode. Therefore, the precise selection of patients and the proper choice of electrode type and direction to treat tumors adjacent to the gallbladder are required to prevent incomplete treatment.
In our study using internally cooled electrodes over a six-year period, the data included a learning curve for the preference of the parallel direction as compared to the perpendicular orientation (38 versus eight). However, we have recently developed a strategy for the use of the parallel direction with internally cooled electrodes and the perpendicular direction with multi-tined electrodes to avoid the potential risk of wall penetration by a needle electrode (11). Incomplete ablation was more frequent for the use of the perpendicular direction in the current study. We think that two possibilities may explain this finding. First, the tip of the straight type electrode might be slightly pulled back due to patient respiration during the ablation. Second, the electrode might be inserted incompletely in cases using the perpendicular direction, due to the risk of gallbladder injury. However, a further study is necessary to confirm these possibilities.
In several investigations for ablation of hepatic tumors adjacent to extrahepatic organs, the reported rates of local tumor progression have varied and have ranged from 2% to 15% (11-14, 18). Our results for local tumor progression (14%) were comparable to results of a prior study using multi-tined expandable electrodes for 53 tumors near the gallbladder (15%) (11). The difference between this study and other previous studies (11, 12) is that the different types of electrodes were used for RF ablation. Although the primary technique effectiveness rates for these two devices showed similar results, we think that the two devices have relative strengths and weaknesses. An expandable electrode may be more suited for ablation of round masses located near the gallbladder because the shape of the zones of necrosis created with this device tends to be spherical (16). This device may also have an advantage in the prevention of tip movement caused by patient respiration during the ablation procedure. However, as the use of an expandable electrode is usually applicable when the electrode can be inserted perpendicularly to the long axis of the gallbladder, a cool-tip electrode may have advantages over the use of an expandable electrode for the ablation of small-sized tumors that abut the gallbladder. In such a situation, these small tumors can be ablated with the cool-tip electrode inserted parallel to the long axis of the gallbladder and positioned off center in the tumor.
This study has the following limitations. First, the study design was retrospective for evaluation of the use of the single RF electrode system. There was no standard protocol (including electrode direction) specific for an HCC near the gallbladder. Second, we used imaging and clinical symptoms as indicators of thermal injury of the gallbladder. Third, there might be measurement errors for the distance between the tumor and the gallbladder wall, as only transverse CT scans were used in this study. Other limitations were that the follow-up periods of the patients were relatively short and the length of follow-up varied.
In conclusion, we have successfully treated 45 patients with 46 HCCs that abutted the gallbladder using an internal cooled RF electrode system. Gallbladder wall thickening was frequently seen, but the wall thickening completely disappeared as seen on subsequent follow-up CT imaging. The therapeutic efficacy of the study was comparable to previous studies (11-13). The direction of electrode insertion (perpendicular), tumor size (≥3 cm) and tumor location (a tumor that abutted the gallbladder) were associated with an increased risk of early incomplete treatment. Therefore, RF ablation of an HCC adjacent to the gallbladder can be performed safely and effectively with proper selection of patients and electrode direction.
Figures and Tables
Fig. 1
Direction of electrode insertion (long arrows). It was categorized as parallel (A) or perpendicular (B) according to direction of electrode tip for gallbladder wall. Distance indicates safety distance between electrode and gallbladder wall.
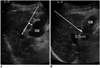
Fig. 2
Imaging findings for 69-year-old woman with residual unablated tumor after radiofrequency ablation where vasovagal syncope occurred during procedure. Electrode was inserted into tumor perpendicular to gallbladder wall.
A. Contrast-enhanced CT scan obtained during arterial phase shows 1.5-cm hepatocellular carcinoma (arrow) abutting gallbladder (double arrows) in liver segment IV.
B. Sagittal sonogram obtained during radiofrequency ablation shows slightly hyperechoic mass (double arrows). Also, note 2-cm active tip (arrow) of electrode between electronic calipers.
C. Contrast-enhanced CT scan obtained immediately after radiofrequency ablation shows residual unablated tumor (arrow) seen as crescent-shape enhancing lesion located at deep portion of ablation zone.
D. Non-enhanced CT scan obtained after transcatheter arterial chemoembolization shows Lipiodol uptake (arrow) by residual unablated tumor.
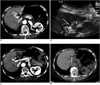
Fig. 3
Imaging findings for 66-year-old woman with hepatocellular carcinoma who had gallbladder wall thickening after radiofrequency ablation are presented. Electrode was inserted into tumor perpendicular to gallbladder wall.
A. Arterial phase CT scan shows 1.6-cm hepatocellular carcinoma (arrow) adjacent to gallbladder in liver segment V. In addition, gallstone (double arrows) is noted.
B. Contrast-enhanced CT scan obtained immediately after radiofrequency ablation shows low-attenuation ablation zone (arrow) larger than tumor. Also, note mild wall thickening of gallbladder adjacent to ablation zone (double arrows).
C. Contrast-enhanced CT scan obtained one month after radiofrequency ablation shows disappearance of gallbladder wall thickening.
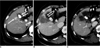
Fig. 4
Imaging findings for 57-year-old man with hepatocellular carcinoma are presented. Electrode was inserted into tumor parallel to gallbladder wall.
A. Arterial phase CT scan shows 2.4-cm hepatocellular carcinoma (arrow) abutting gallbladder in liver segment V.
B. Immediate follow-up CT image after radiofrequency ablation show low-attenuation ablation zone (arrow) and mild focal wall thickening of gallbladder (double arrows).
C. Contrast-enhanced CT scan obtained one month after radiofrequency ablation shows disappearance of gallbladder wall thickening.
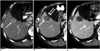
Fig. 5
Imaging findings for 59-year-old man with local tumor progression after radiofrequency ablation are presented. Electrode was inserted into tumor parallel to gallbladder wall.
A. Contrast-enhanced CT scan obtained during arterial phase shows 3.0-cm hepatocellular carcinoma (arrow) adjacent to gallbladder in liver segment V.
B. Oblique sonogram obtained during radiofrequency ablation shows hypoechoic mass adjacent to gallbladder. Single ablation with 3-cm single straight electrode (arrows) was performed for 12 minutes. Also, note 3-cm active tip of electrode between electronic calipers.
C. Contrast-enhanced CT scan obtained immediately after radiofrequency ablation shows low-attenuation ablation zone with peripheral hyperemia (arrow).
D. Contrast-enhanced CT scan obtained three months after radiofrequency ablation shows nodular enhancing lesion (arrow) at inferomedial aspect of ablation zone that is indicative of local tumor progression.
References
1. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005. 129:122–130.
2. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–240.
3. Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002. 223:331–337.
4. Catalano O, Esposito M, Nunziata A, Siani A. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdom Imaging. 2000. 25:607–614.
5. Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol. 2001. 31:322–326.
6. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006. 243:321–328.
7. Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005. 39:247–252.
8. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.
9. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.
10. Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: imaging spectrum and management. Radiographics. 2005. 25:S57–S68.
11. Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006. 17:671–683.
12. Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008. 33:428–436.
13. Chopra S, Dodd GD 3rd, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003. 180:697–701.
14. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in socalled high-risk locations. Hepatology. 2006. 43:1101–1108.
15. Ng KK, Lam CM, Poon RT, Shek TW, Yu WC, To JY, et al. Porcine liver: morphologic characteristics and cell viability at experimental radiofrequency ablation with internally cooled electrodes. Radiology. 2005. 235:478–486.
16. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
17. Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radiofrequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001. 221:447–454.
18. Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, et al. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. AJR Am J Roentgenol. 2004. 183:1417–1424.
19. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005. 16:765–778.
20. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134.
21. Lee J, Rhim H, Jeon YH, Lim HK, Lee WJ, Choi D, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol. 2008. 190:418–425.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download