Abstract
We report here two cases of foreign body granulomas that arose from the pelvic wall and liver, respectively, and simulated recurrent colorectal carcinomas in patients with a history of surgery. On contrast-enhanced CT and MR images, a pelvic wall mass appeared as a well-enhancing mass that had invaded the distal ureter, resulting in the development of hydronephrosis. In addition, a liver mass had a hypointense rim that corresponded to the fibrous wall on a T2-weighted MR image, and showed persistent peripheral enhancement that corresponded to the granulation tissues and fibrous wall on dynamic MR images. These lesions also displayed very intense homogeneous FDG uptake on PET/CT.
Approximately 37-44% of patients who undergo surgical resection to treat colorectal cancer develop recurrence and/or a metastasis (1). Thus, postoperative surveillance for early detection of a recurrence is important to the patient's survival. Various imaging modalities play an important role in the preoperative staging and postoperative surveillance of colorectal cancer (1, 2). However, it has been reported that foreign body granulomas, which can occur in any location of the body, can cause imaging pitfalls in patients undergoing postoperative surveillance for tumor recurrence (3-7). Here, we describe the computed tomography (CT), magnetic resonance imaging (MRI) and F-18 fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT imaging findings of two cases of foreign body granulomas that mimicked recurrent colorectal carcinomas.
A 67-year-old man underwent Hartmann's procedure for rectal cancer in April 2005. At that time, the pathologic staging of the rectal cancer was proper muscle invasion without lymph node metastasis (T2 N0 M0). Following surgery, periodic surveillance was performed with the use of contrast-enhanced CT and PET/CT imaging. Thirty months later, a CT scan demonstrated the presence of a 3.4-cm ovoid, well-enhancing mass at the right pelvic side wall that had invaded the right distal ureter, resulting in hydronephrosis (Fig. 1A). The T1-weighted and fat-saturated T2-weighted MR images revealed that the mass had an isointense and hyperintense signal intensity, respectively, when compared to the signal intensity of the adjacent muscle. Furthermore, the mass demonstrated homogeneous enhancement following intravenous injection of gadobenate dimeglumine (MultiHance, Bracco, Milan, Italy) (Fig. 1B-D). Seventeen days after the CT scan, a PET/CT scan was performed 60 minutes after the injection of 9.24 mCi F-18 FDG. The PET/CT images demonstrated focal, intense and homogeneous FDG uptake in the mass at the right pelvic wall (Fig. 1E), with a maximum standard uptake value (SUVmax) of 9.1 being observed. A review of serial follow-up PET/CT images obtained six months earlier revealed a 1.6-cm mass with a SUVmax of 3.5 that was missed during the initial evaluation (Fig. 1E). Taken together, these findings suggested a diagnosis of a recurrent tumor. Accordingly, an excisional biopsy was performed to confirm this diagnosis. Based on the histological findings, a diagnosis of suture granuloma was made (Fig. 1F).
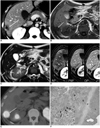
A 37-year-old man was admitted to our hospital for resection of a presumed liver metastasis. Three months earlier, the patient had undergone a right hemicolectomy for transverse colon cancer and a wedge resection for a solitary metastasis in Couinaud's segment VI of the liver. At that time, the TNM classification for the pathologic staging of colon cancer was T4 (serosal invasion), N1 (three regional lymph node metastasis), and M1 (liver metastasis). A CT scan performed prior to the current admission revealed a small ovoid, well defined, hypodense mass with peripheral contrast enhancement in the subcapsular region of segment VI adjacent to the previous resection site (Fig. 2A). These CT findings suggested a liver metastasis. On the T1-weighted MR images, the mass was hypointense relative to the liver parenchyma, while it was hyperintense on the T2-weighted MR images. In addition, the T2-weighted MR image revealed a hypointense rim at the peripheral portion of the hyperintense mass (Fig. 2B, C). Furthermore, the mass showed persistently peripheral enhancement on the gadolinium-enhanced MR images (Fig. 2D). Two weeks after the CT scan, a PET/CT was performed to evaluate other occult metastases, including this lesion. The results of the PET/CT scan revealed the presence of a solitary liver mass with markedly increased accumulation of FDG (SUVmax, 9.6) (Fig. 2E). The patient underwent a partial resection of the liver for the mass. Histology of the resected mass revealed a localized necrosis with a foreign body granuloma surrounded by a fibrous wall (Fig. 2F). When correlated with the imaging findings, the central necrotic areas and the granulation tissues combined with the peripheral fibrous wall corresponded to areas of low attenuation and peripheral enhancement, respectively, on the CT images and to areas of hypointensity and peripheral enhancement, respectively, on the gadolinium-enhanced MR images (Fig. 2A, D). The outer fibrous wall was especially well visualized as a peripheral hypointense rim on the T2-weighted MR image (Fig. 2C).
Foreign body materials that can induce a foreign body reaction include non-absorbable suture materials, surgical sponges, Teflon and activated charcoal (4-8). These lesions are of clinical importance because they create imaging findings that may be confused with a malignant lesion, which can lead to unnecessary surgical treatment.
Although various appearances may present, CT findings of retained surgical sponges, which are one of the most common causative materials, have been described as a typical spongiform pattern with gas bubbles (8). Most suture granulomas reported have been associated with inguinal herniorrhaphy, and manifest as paravesical suture granulomas (9, 10). Suture granulomas are often seen as irregular masses with central necrosis that are in contact with the bladder wall, simulating a bladder carcinoma (9, 10). The only reported case of a suture granuloma unrelated to herniorrhaphy in the abdomen was a small nodule with increased FDG activity in the mesentery that was found on a PET/CT scan of a patient who had undergone surgery for colon cancer (3). The suture granuloma described in Case 1 presented as a growing mass in the pelvic wall that showed homogeneous contrast enhancement and marked accumulation of FDG and caused hydronephrosis by direct invasion of the distal ureter. The above imaging findings, which had an appearance similar to that of lymph node metastasis, led us to make a diagnosis of recurrent rectal cancer. In general, the diagnosis of recurrent malignant disease is strongly suggested when CT findings such as the enlargement of a soft-tissue mass over time, enlarging regional lymphadenopathy, and invasion of contiguous structures are observed during the postoperative surveillance of colorectal carcinomas (1). However, the imaging findings of suture granuloma in Case 1 indicate that the above-described findings may not be specific enough for a diagnosis of a recurrent tumor to be made. Therefore, a surgical confirmation or biopsy is mandatory for accurate diagnosis, especially when the primary tumor is limited to the bowel wall or masses develop two years after the initial operations, as occurred in our case.
Imaging appearances of foreign body granulomas that have previously been reported in the clinical literature are frequently described as peripheral enhancing masses on contrast-enhanced CT scans or MR images and ring-shaped FDG uptakes on PET/CT scans (4, 8-11). These appearances represent the wall formation induced by a fibroblastic reaction. Similar radiological findings were observed in Case 2. However, PET/CT imaging in Case 2 demonstrated homogeneous FDG uptake within the lesion instead of a ring-shaped uptake. Considering that the sizes of the masses in which the ring-shaped FDG uptakes were reported were relatively large (8, 11), the small size observed in the current case may explain the discrepancy between the imaging modalities. Furthermore, it has been reported that focal homogeneous FDG uptake by small foreign body granulomas can be induced by suture material, activated charcoal, and Teflon (3, 5-7).
In Case 2, the clinical history may be the key to a diagnosis of a foreign body granuloma. This is because the lesion occurred shortly (3 months) after the first surgery and was located close to the resection site of a hepatic metastasis without demonstration of other metastatic foci. However, because the imaging findings were similar to metastasis, it was not possible to diagnose this lesion preoperatively. In Case 2, the mass had a peripheral hypointense rim that corresponded to the fibrous wall on the T2-weighted MR image. In addition, the mass displayed peripheral enhancement that corresponded to the granulation tissues and peripheral fibrous wall, beginning in the arterial phase and persisting into the delayed phase on the contrast-enhanced MRI. Outwater et al. (12) reported that peripheral hypointense rims on T2-weighted MR images were observed in 25% of the hepatic colorectal metastases, and that this represented pathologic changes in the surrounding hepatic parenchyma such as compressed hepatic parenchyma, hepatocellular atrophy, fibrosis, and congested sinusoids. However, they did not perform enhancement imaging. Another MR study for liver metastases revealed peripheral ring enhancement persisting through all phases of enhancement in all hypovascular metastases from colon adenocarcinoma, which corresponded pathologically to a thin zone containing fibrous and inflammatory cells (13). However, in that case, the peripheral ring enhancement was seen as a peripheral hyperintense rim on the T2-weighted MR images. Thus, the combination of imaging features observed on the T2-weighted and gadolinium-enhanced MR images in our case may be a helpful clue that enables differentiation of a foreign body granuloma from a hepatic metastasis.
It should be noted that very intense FDG uptakes within the foreign body granulomas (SUVmax > 9.0) were observed in these two cases. Although false positive FDG uptake has been observed in various benign conditions, including inflammation, granulomatous disease, and abscess (3, 5), such intense uptakes make diagnosis more challenging. Indeed, Metser et al. (14) reported that 56% of benign nonphysiologic lesions with increased FDG uptake displayed moderate or marked FDG uptake. Furthermore, they reported that CT characterization improves the specificity of PET/CT reporting in such cases. However, foreign body granulomas were not included in their study population.
In summary, we present two cases of foreign body granulomas that mimicked recurrent colorectal carcinomas. One case demonstrated a well-enhancing mass with invasion of the distal ureter arising from the pelvic side wall that developed two years after primary surgery. The other case showed a peripheral enhancing mass at the hepatic resection site for a metastasis that occurred three months after primary surgery. Intense homogeneous FDG uptakes were observed in both cases, which presented a difficult diagnostic challenge.
These cases demonstrate that foreign body granulomas can manifest as a growing mass with invasion of contiguous structures; therefore, careful attention should be paid to any suspected recurrent tumor, even if they have imaging features that are considered to be typical of a recurrent tumor. In foreign body granuloma that develops in the liver, the MR imaging finding of a peripheral hypointense rim on the T2-weighted image combined with persistent peripheral enhancement on dynamic MR images may be helpful in the diagnosis of this uncommon lesion. Radiologists and surgeons should be aware of the possible occurrence of foreign body granulomas during postoperative surveillance for colorectal carcinomas.
Figures and Tables
Fig. 1
67-year-old man with suture granuloma.
A. Contrast-enhanced axial CT scan shows ovoid, well-enhancing mass attached to right pelvic side wall (arrows).
B, C. This mass shows isointense and hyperintense signal intensity relative to adjacent muscle on T1-weighted (black arrows in B) and fat-saturated T2-weighted MR images (white arrows in C), respectively.
D. Coronal gadolinium-benzyloxypropionictetraacetate (Gd-BOPTA) enhanced T1-weighted MR image shows homogeneously enhancing mass (arrow) that invades right distal ureter (double arrows).
E. Axial PET/CT fusion image reveals focal FDG uptake in right pelvic side wall (arrow in left). PET/CT fusion image obtained six months later demonstrates interval growth of lesion with intense FDG uptake (arrow in right).
F. Photomicrograph of specimen obtained from excisional biopsy shows suture material (double arrows and inset) and multinucleated giant cells (black arrows) (Hematoxylin & Eosin staining, ×400).
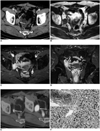
Fig. 2
37-year-old man with foreign body granuloma in liver.
A. Contrast-enhanced axial CT scan obtained during portal venous phase demonstrates well-circumscribed, ovoid hypodense mass with peripheral enhancement in segment VI of liver (arrow).
B, C. This mass is hypointense relative to liver on T1-weighted MR image (arrow in B) and hyperintense on T2-weighted MR image (arrow in C). Hypointense rim (double arrows in C) is present at peripheral portion of mass on T2-weighted MR image.
D. Serial gadolinium-benzyloxypropionictetraacetate (Gd-BOPTA) dynamic MR images show persistently peripheral enhancement of mass (black arrows) from arterial (AP) to delayed phases (DP). PVP = portal-venous phase
E. Axial PET/CT fusion image reveals intense homogeneous uptake (arrow).
F. Histological examination (Hematoxylin & Eosin staining, ×40) reveals that mass has thin, fibrous wall (F) and contains granulation tissues with inflammatory cells (G) and necrotic areas (N). L = normal liver. High-power photomicrograph (inset, Hematoxylin & Eosin staining, ×200) shows foreign body granuloma composed of multiple multinucleated giant cells (black arrows). White arrow indicates foreign body material that has been engulfed by giant cell.
References
1. Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: imaging features and role in management. Radiographics. 2000. 20:419–430.
2. Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005. 23:8512–8519.
3. Lim JW, Tang CL, Keng GH. False positive F-18 fluorodeoxyglucose combined PET/CT scans from suture granuloma and chronic inflammation: report of two cases and review of literature. Ann Acad Med Singapore. 2005. 34:457–460.
4. Poyanli A, Bilge O, Kapran Y, Güven K. Case report: foreign body granuloma mimicking liver metastasis. Br J Radiol. 2005. 78:752–754.
5. Chung YE, Kim EK, Kim MJ, Yun M, Hong SW. Suture granuloma mimicking recurrent thyroid carcinoma on ultrasonography. Yonsei Med J. 2006. 47:748–751.
6. Kim YK, Park HS. Foreign body granuloma of activated charcoal. Abdom Imaging. 2008. 33:94–97.
7. Yeretsian RA, Blodgett TM, Branstetter BF 4th, Boberts MM, Meltzer CC. Teflon-induced granuloma: a false-positive finding with PET resolved with combined PET and CT. AJNR Am J Neuroradiol. 2003. 24:1164–1166.
8. Ghersin E, Keidar Z, Brook OR, Amendola MA, Engel A. A new pitfall on abdominal PET/CT: a retained surgical sponge. J Comput Assist Tomogr. 2004. 28:839–841.
9. Carroll KM, Sairam K, Olliff SP, Wallace DM. Case report: paravesical suture granuloma resembling bladder carcinoma on CT scanning. Br J Radiol. 1996. 69:476–478.
10. Kise H, Shibahara T, Hayashi N, Arima K, Yanagawa M, Kawamura J. Paravesical granuloma after inguinal herniorrhaphy. Case report and review of the literature. Urol Int. 1999. 62:220–222.
11. Nakajo M, Jinnouchi S, Tateno R, Nakajo M. 18F-FDG PET/CT findings of a right subphrenic foreign-body granuloma. Ann Nucl Med. 2006. 20:553–556.
12. Outwater E, Tomaszewski JE, Daly JM, Kressel HY. Hepatic colorectal metastases: correlation of MR imaging and pathologic appearance. Radiology. 1991. 180:327–332.
13. Danet IM, Semelka RC, Leonardou P, Braga L, Vaidean G, Woosley JT, et al. Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol. 2003. 181:809–817.
14. Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR Am J Roentgenol. 2007. 189:1203–1210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download