Abstract
Recent human genetic studies have shown that Gβ5 is related to various clinical symptoms, such as sinus bradycardia, cognitive disability, and attention deficit hyperactivity disorder. Although the calcium signaling cascade is closely associated with a heterotrimeric G-protein, the function of Gβ5 in calcium signaling and its relevance to clinical symptoms remain unknown. In this study, we investigated the in vitro changes of store-operated calcium entry (SOCE) with exogenous expression of Gβ5. The cells expressing Gβ5 had enhanced SOCE after depletion of calcium ion inside the endoplasmic reticulum. Gβ5 also augmented Stim1- and Orai1-dependent SOCE. An ORAI1 loss-of-function mutant did not show inhibition of Gβ5-induced SOCE, and a STIM1-ERM truncation mutant showed no enhancement of SOCE. These results suggested a novel role of GNB5 and Stim1, and provided insight into the regulatory mechanism of SOCE.
Before the discovery of direct activation and interaction of Gβγ with the potassium channel in the heart [1], the Gα subunit was considered the only effector, and the Gβγ complex was considered a signal inhibitor molecule. However, after the discovery of several biological effects of Gβγ, many studies have attempted to understand its functions. It is currently known that the Gβγ complex has its own properties and functional roles in cellular signaling pathways [23]. Gβγ has specific tissue expression patterns, and specific coupling between the GPCRs [3]. For example, a study showed that Gβ1γ1 assisted Gαt binding to rhodopsin, but Gβ1γ2 did not [4]. Gα13β1γ3 complexes are coupled with the angiotensin receptor (AT1A) in rat portal vein myocytes, and enhance cytosolic calcium ion transport [5]. These previous studies as well as others suggest that each component of the Gβγ subunit complex has its own unique role in specific tissues.
Interestingly, recent genetic studies have reported that mutations of GNB5 (the gene encoding Gβ5) were associated with the symptoms of sinus bradycardia, cognitive disability [6], and attention deficit hyperactivity disorder (ADHD) [78] in the human. Moreover, a mouse model that lacked Gβ5 showed hyperactivity [9].
GNB5 related symptom related with calcium signaling. Calcium signaling is closely related with cardiac and neural function. In particular, store-operated calcium entry (SOCE) plays important roles in the heart [101112]. SOCE allows replenishment of sarcoplasmic reticulum calcium in the cardiomyocytes; therefore, defects in SOCE lead to some cardiac diseases [1213]. In neurons, calcium signaling is also important. Spontaneously hypertensive rats, as an animal model of ADHD [14], have a dysfunction of calcium signaling [1516] and lower brain Ca2+-ATPase activity [17].
Consequently, previous studies have implied a relationship of the GNB5 gene and SOCE. However, there are no studies describing this relationship, and therefore we investigated the regulatory mechanism of GNB5-induced effects on SOCE.
The genes, GNB1 (NCBI accession number: NM_002074), GNB5 (NCBI accession number: NM_006578), GNG11 (NCBI accession number: NM_004126), and GNG13 (NCBI accession number: NM_016541), were cloned into the mammalian expression vectors, pEGFP-N1 and p3XFLAG-CMV14. The mutant Orai1R91W [1819] and ΔERM-Stim1D76A [2021] were also used. All the samples were incubated for 24 h after transfection using Lipofectamine 2,000 (Invitrogen, Carlsbad, CA, USA).
HEK293T cells were maintained in high-glucose Dulbecco's Modified Eagle's Medium with 10% fetal bovine serum and 1× antibiotic-antimycotic reagent (Gibco, Gaithersburg, MD, USA) in 95% CO2, 5% O2, at 37℃. Subculturing was done with trypsin/EDTA when the cells reached confluency. The cells used in the calcium imaging experiments were cultured on 24 mm×24 mm glass slides.
The cells were cultured on 24 mm×24 mm glass slides at a density of 1.5×106 cells/ml for 48 h and then treated with 4 µM Fura-2/AM and 0.05% pluronic acid F-127 for 30 min in physiological salt solution at room temperature. Fura-2/AM fluorescence was measured at an excitation wavelength of 340/380 nm, and an emission wavelength of 510 nm (expressed as the ratio=F340/F380), using an imaging system (Molecular Devices, Sunnyvale, CA, USA). The emitted fluorescence was monitored using a CCD camera (CoolSNAP, Tucson, AZ, USA) attached to an inverted microscope. Fluorescence images were obtained at 2-s intervals. All data were analyzed using MetaFluor software (Molecular Devices, Downingtown, PA, USA). Intracellular calcium is shown as representative traces. The data represent the mean±S.E.M. ΔPlateau was defined as the value that difference of the Fura-2/AM ratio between the injection point of 2 mM Ca2+ solution and highest value.
To investigate the role of the Gβ and Gγ subunits in SOCE, we overexpressed GNB1, GNB5, GNG11, and GNG12 in HEK293T cells. After the expression of each protein subunit was confirmed, we measured the amount of SOCE (Fig. 1A). To maximize SOCE, we used 25 µM cyclopiazonic acid (CPA), and induced calcium entry using 2 mM Ca2+ physiological salt solution. SOCE was increased after exogenous overexpression of the GNB5 gene (Fig. 1B), suggesting that Gβ5 protein affects the SOCE in HEK293T cells.
Because Stim1-Orai1 complexes are known as ubiquitous store-operated Ca2+ channels [22], we investigate SOCE after co-expression of Stim1, Orai1, and Gβ5. SOCE was significantly increased when all the components were expressed (Fig. 2B). With Gβ5 expression, the SOCE was approximately 2.5- fold higher than that with Stim1 or Orai1 expression. SOCE was also enhanced in Stim1-Gβ5 expressing cells and in Orai1-Gβ5 expressing cells. These results suggested that Gβ5 interacts with Orai1 or Stim1, or both.
To confirm the target of Gβ5, we co-expressed Orai1R91W, a loss-of-function mutant of Orai1, with Gβ5. Although mutant Orai1 was expressed, SOCE was still enhanced by Gβ5 expression (Fig. 3B). This might be a result of the presence of endogenous Orai1 and its participation in SOCE.
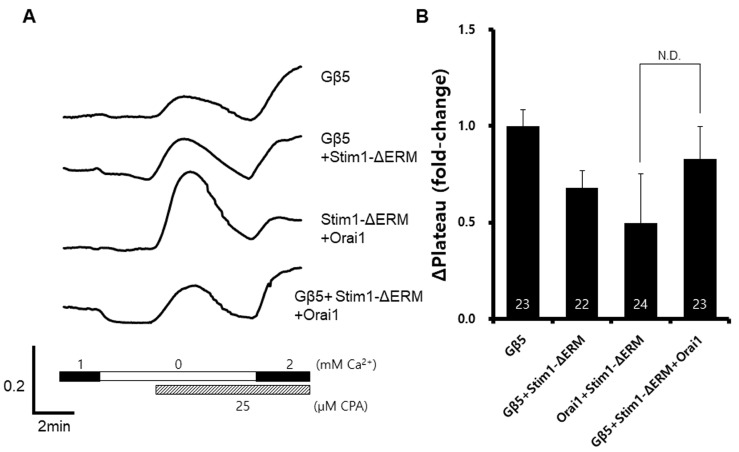
ΔERM-Stim1D76A is a dominant-negative form of STIM1 (DN-STIM1). When we expressed DN-STIM1 with GNB5, the enhancement of SOCE was diminished (Fig. 4B). This result supported the hypothesis that GNB5 may interact with STIM1.
GPCR signaling is a well-established pathway by which intra-cellular signals interact with extracellular signals [23]. Coupling between phospholipase Cβ (PLCβ) induces inositol-1, 4, 5-triphosphate (IP3) generation and subsequent cytosolic Ca2+ ion cascades [24]. Specific Gβγ subunit complexes activate PLCβ and cause its translocation to the cytosol, to subsequently interact with the IP3 receptor in the endoplasmic reticulum (ER), and induce changes in Ca2+ conductance [2526]. Evidence also suggests a direct activation of IP3 by Gβγ complexes [27]. However, the role of Gβγ complexes in SOCE remains unknown. From our present results, we concluded that Gβ5 expression enhanced SOCE (Fig. 1B).
The mechanism by which Gβ5 increased SOCE remains unknown. Stim1-Orai1 dependent SOCE was significantly increased by the expression of Gβ5 (Fig. 2B). The replacement of wild-type Orai1 with a loss-of-function Orai1 mutant, Orai1R91W, did not alter SOCE. However, a loss-of-function Stim1 mutant, ΔERM-Stim1D76A, inhibited the SOCE induced by Gβ5. These results suggested that GNB5 gene expression may affect Stim1 action, however, whether this is a direct or indirect relationship is unclear. Further studies are needed to evaluate the interactions of Gβ5 with Stim1.
The Stim1 protein has an EF-hand domain for calcium sensing at the ER lumen [28]. The cytosolic portion of Stim1 begins with an ERM domain that starts with a coiled-coil domain for oligomerization and oligomer-induced recruitment of Orai1 protein to the plasma membrane [19]. In the ERM domain of the Stim1 protein, the Stim1 Orai1 activating region (SOAR) performs key functions in the activation of Orai1 [1929]. In our study, ΔERM-Stim1D76A inhibited the SOCE induced by Gβ5. Gβ5 also has α-helical extension of 20 amino acids forms a coiled-coil domain [30]. This suggests that the coiled-coil domain may enhance the recruitment of the Orai1 protein.
Many Stim1-modulating molecules have been identified. For example, SARAF and TEM66 bind to the Stim1 SOAR domain to cause reduced Orai1 activity [31]. STIMATE and TEM110 interact with Stim1 to promote its conformational switch changes [32]. Cracm1, calcium related activating current modulator 1, is a plasma membrane protein that is essential for SOCE [33]. The R7 RGS protein, regulator of G-protein signaling protein, mediates Gβ5 neuronal signaling [89]. Similarly, GNB5 may act by modulating molecules related to STIM1.
The TRPC pathway is another important pathway of SOCE control in mammalian cells [192934]. In a mouse model that lacks Trpc3, reduced SOCE in pancreatic acinar cells and reduced amylase secretion were observed [35]. Stim1 SOAR domains are also important for activation of Trpc3 [19]. Previous studies indicated that Gβ5 interacts with Stim1, and its expression promotes Trpc3-dependent SOCE. Studies of SOCE in Gβ5-Trpc3 expressing cells, compared with cells expressing Trpc3 alone, have not been reported. Therefore, the role of Gβ5 and Stim1 should be further studied.
The mechanism and function of the Gβγ complexes still remain to be determined. Gβγ complexes are potential therapeutic targets, therefore, our findings may contribute to the development of novel approaches for cures of diseases.
ACKNOWLEDGEMENTS
Thank you for the generous gift, Orai1R91W clone, from the Dr. Jeong Hee Hong, in the Department of Physiology, College of Medicine, Gachon University, Korea. Thank you for the generous gift, ΔERM-Stim1D76A clone, from the Dr. Joo Young Kim, Department of Pharmacology, College of Medicine, Yonsei University, Korea. This article was supported by a faculty research grant of Yonsei University College of Dentistry (6-2016-0026).
Notes
References
1. Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987; 325:321–326. PMID: 2433589.
2. Dupré DJ, Robitaille M, Rebois RV, Hébert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009; 49:31–56. PMID: 18834311.
3. Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008; 65:2191–2214. PMID: 18488142.

4. Kisselev O, Ermolaeva M, Gautam N. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. J Biol Chem. 1995; 270:25356–25358. PMID: 7592699.
5. Macrez-Leprêtre N, Kalkbrenner F, Morel JL, Schultz G, Mironneau J. G protein heterotrimer Galpha13beta1gamma3 couples the angiotensin AT1A receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J Biol Chem. 1997; 272:10095–10102. PMID: 9092554.
6. Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, Lahrouchi N, Guex N, Napolioni V, Tessadori F, Beekman L, Nannenberg EA, Boualla L, Blom NA, de Graaff W, Kamermans M, Cocciadiferro D, Malerba N, Mandriani B, Akdemir ZHC, Fish RJ, Eldomery MK, Ratbi I, Wilde AAM, de Boer T, Simonds WF, Neerman-Arbez M, Sutton VR, Kok F, Lupski JR, Reymond A, Bezzina CR, Bakkers J, Merla G. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am J Hum Genet. 2016; 99:704–710. PMID: 27523599.

7. Shamseldin HE, Masuho I, Alenizi A, Alyamani S, Patil DN, Ibrahim N, Martemyanov KA, Alkuraya FS. GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition. Genome Biol. 2016; 17:195. PMID: 27677260.

8. Xie K, Allen KL, Kourrich S, Colón-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010; 13:661–663. PMID: 20453851.
9. Xie K, Ge S, Collins VE, Haynes CL, Renner KJ, Meisel RL, Lujan R, Martemyanov KA. Gβ5-RGS complexes are gatekeepers of hyper-activity involved in control of multiple neurotransmitter systems. Psychopharmacology (Berl). 2012; 219:823–834. PMID: 21766168.

10. Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol. 2013; 305:H446–H458. PMID: 23792674.

11. Kar P, Parekh A. STIM proteins, Orai1 and gene expression. Channels (Austin). 2013; 7:374–378. PMID: 23765192.

12. Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr. 2013; 71:209–235. PMID: 23890117.
13. Shaikh S, Troncoso R, Criollo A, Bravo-Sagua R, García L, Morselli E, Cifuentes M, Quest AF, Hill JA, Lavandero S. Regulation of cardiomyocyte autophagy by calcium. Am J Physiol Endocrinol Metab. 2016; 310:E587–E596. PMID: 26884385.

14. Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005; 1:9. PMID: 16022733.

15. Lehohla M, Kellaway L, Russell VA. NMDA receptor function in the prefrontal cortex of a rat model for attention-deficit hyperactivity disorder. Metab Brain Dis. 2004; 19:35–42. PMID: 15214504.

16. Lehohla M, Russell V, Kellaway L. NMDA-stimulated Ca2+ uptake into barrel cortex slices of spontaneously hypertensive rats. Metab Brain Dis. 2001; 16:133–141. PMID: 11769326.
17. Horn JL, Janicki PK, Franks JJ. Diminished brain synaptic plasma membrane Ca2+-ATPase activity in spontaneously hypertensive rats: association with reduced anesthetic requirements. Life Sci. 1995; 56:PL427–PL432. PMID: 7746091.
18. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008; 105:2895–2900. PMID: 18287061.

19. Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010; 584:2022–2027. PMID: 19944100.

20. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005; 15:1235–1241. PMID: 16005298.
21. Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006; 8:1003–1010. PMID: 16906149.
22. Hogan PG, Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochem Biophys Res Commun. 2015; 460:40–49. PMID: 25998732.

23. Nieto Gutierrez A, McDonald PH. GPCRs: Emerging anti-cancer drug targets. Cell Signal. 2018; 41:65–74. PMID: 28931490.

24. Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009; 1793:933–940. PMID: 19010359.

25. Myung CS, Garrison JC. Role of C-terminal domains of the G protein beta subunit in the activation of effectors. Proc Natl Acad Sci U S A. 2000; 97:9311–9316. PMID: 10922079.

26. Rebres RA, Roach TI, Fraser ID, Philip F, Moon C, Lin KM, Liu J, Santat L, Cheadle L, Ross EM, Simon MI, Seaman WE. Synergistic Ca2+ responses by Gai- and Gaq-coupled G-protein-coupled receptors require a single PLCb isoform that is sensitive to both Gβγ and Gαq. J Biol Chem. 2011; 286:942–951. PMID: 21036901.
27. Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol. 2003; 13:872–876. PMID: 12747838.
28. Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C. Recent progress on STIM1 domains controlling Orai activation. Cell Calcium. 2009; 46:227–232. PMID: 19733393.

29. Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009; 11:337–343. PMID: 19182790.

30. Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996; 379:369–374. PMID: 8552196.
31. Cao X, Choi S, Maléth JJ, Park S, Ahuja M, Muallem S. The ER/PM microdomain, PI(4,5)P2 and the regulation of STIM1-Orai1 channel function. Cell Calcium. 2015; 58:342–348. PMID: 25843208.

32. Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong MQ, Walker CL, Hogan PG, Wang Y, Zhou Y. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol. 2015; 17:1339–1347. PMID: 26322679.
33. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006; 312:1220–1223. PMID: 16645049.
34. Shin DM, Son A, Park S, Kim MS, Ahuja M, Muallem S. The TRPCs, Orais and STIMs in ER/PM Junctions. Adv Exp Med Biol. 2016; 898:47–66. PMID: 27161224.

35. Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011; 140:2107–2115. 2115.e1–2115.e4. PMID: 21354153.
Fig. 1
Enhanced store-operated Ca2+ entry induced by overexpression of GNB5
After depletion of ER Ca2+ stores using cyclopiazonic acid and EGTA, Ca2+ entry was measure by Fura2-AM fluorescent signal ratios. A representative trace (A) summarizes the data (B), and the number inside the column is the total cell number from multiple independent experiments (n=3-4). Data were expressed as the mean±SEM. *p<0.05 compared with GFP.
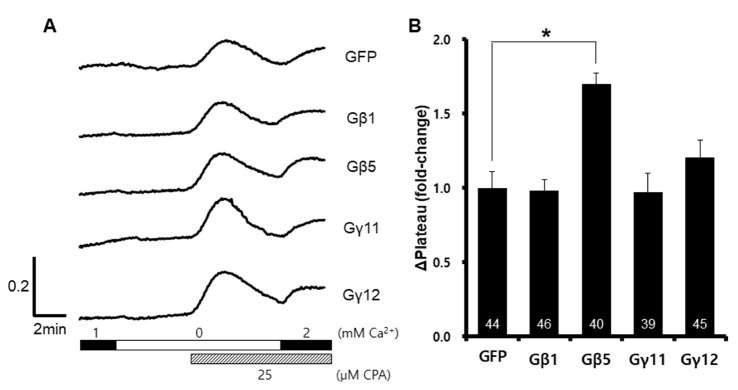
Fig. 2
Increased Stim1-Orai1 dependent store-operated Ca2+ entry after GNB5 overexpression.
Gβ5, Stim1, and Orai1 were co-expressed in HEK293T cells. A representative trace (A) summarizes the data (B), and the number inside the column is the total cell number from multiple independent experiments (n=3-4). Data were expressed as the mean±SEM. *p<0.05 compared with control.
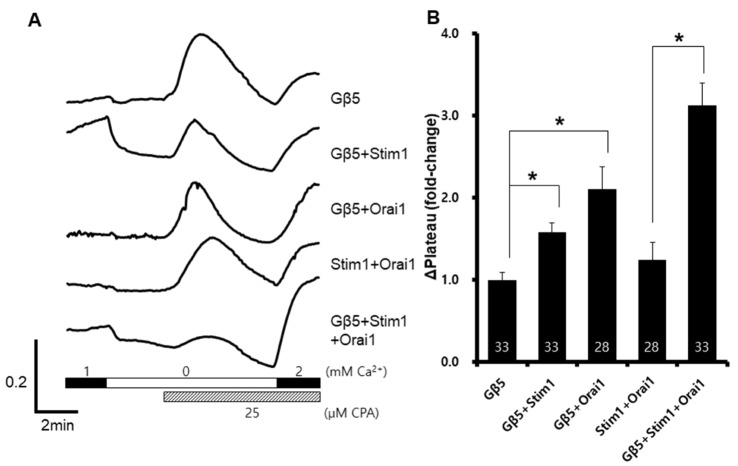
Fig. 3
Effects of a loss-of-functional Orai1 mutant on store-operated Ca2+ entry.
Wild-type Gβ5, wild-type Stim1, and Orai1R91W were co-expressed in HEK293T cells. A representative trace (A) summarizes the data (B), and the number inside the column is the total cell number from multiple independent experiments (n=3-4). Data were expressed as the mean±SEM. *p<0.05 compared with control.
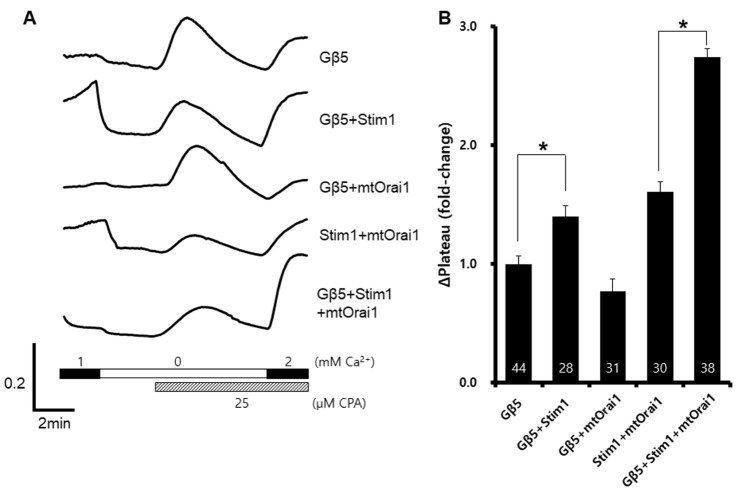
Fig. 4
Diminished enhancement of the store-operated Ca2+ entry induced by Gβ5 and the co-expression of a dominant-negative Stim1.
Wild-type Gβ5, ΔERM-Stim1D76A, and wild-type Orai1 were co-expressed in HEK293T cells. A representative trace (A) summarizes the data (B), and the number inside the column is the total cell number from multiple independent experiments (n=3-4). N. D.=not determined.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download