Abstract
The aim of the present study was to examine the effects of preemptive analgesia on the development of trigeminal neuropathic pain. For this purpose, mechanical allodynia was evaluated in male Sprague-Dawley rats using chronic constriction injury of the infraorbital nerve (CCI-ION) and perineural application of 2% QX-314 to the infraorbital nerve. CCI-ION produced severe mechanical allodynia, which was maintained until postoperative day (POD) 30. An immediate single application of 2% QX-314 to the infraorbital nerve following CCI-ION significantly reduced neuropathic mechanical allodynia. Immediate double application of QX-314 produced a greater attenuation of mechanical allodynia than a single application of QX-314. Immediate double application of 2% QX-314 reduced the CCI-ION-induced upregulation of GFAP and p-p38 expression in the trigeminal ganglion. The upregulated p-p38 expression was co-localized with NeuN, a neuronal cell marker. We also investigated the role of voltage-gated sodium channels (Navs) in the antinociception produced by preemptive application of QX-314 through analysis of the changes in Nav expression in the trigeminal ganglion following CCI-ION. Preemptive application of QX-314 significantly reduced the upregulation of Nav1.3, 1.7, and 1.9 produced by CCI-ION. These results suggest that long-lasting blockade of the transmission of pain signaling inhibits the development of neuropathic pain through the regulation of Nav isoform expression in the trigeminal ganglion. Importantly, these results provide a potential preemptive therapeutic strategy for the treatment of neuropathic pain after nerve injury.
Acute pain has been strongly correlated with chronic postoperative pain in clinical studies [123]. These studies have demonstrated that the incidence and severity of chronic postoperative pain can be predicted through experimental pain assessment. In addition, the findings of these studies indicate that a new strategy, involving preemptive analgesia, may reduce the postoperative pain arising from surgical wounds and thereby treating postoperative chronic pain. This concept is supported by clinical reports that preoperative regional anesthesia or epidural opioid analgesia significantly can reduce subsequent acute postoperative pain by blocking the sensitizing effects of surgical stimulation [45]. The ability of preemptive analgesia to decrease postoperative pain has also been demonstrated in animal studies. Preemptive epidural administration of the local anesthetic bupivacaine with morphine effectively produces analgesia in dogs and cats that is longer-lasting than the analgesic effects of morphine alone [6]. In addition, preemptive treatment with 2% lidocaine reduces the postoperative neuropathic pain associated with sciatic nerve compression [7]. These results suggest that preemptive analgesia, or early pain control, attenuates the progression of chronic pain caused by surgical lesions. However, the mechanisms underlying the antinociceptive effects of preemptive analgesia in trigeminal neuropathic pain remain unclear.
QX-314, a membrane-impermeable quaternary lidocaine derivative, has no effect on neuronal sodium channels on extracellular application. However, co-administration of QX-314 with capsaicin, a transient receptor potential (TRP) V1 receptor agonist, produces long-lasting sensory-selective blockage [8910]. An activation of TRP V1 channels by capsaicin allows QX-314 to enter TRP V1-positive neurons, producing an analgesic effect by blocking sodium channels intracellularly [891112]. However, no studies have evaluated the effects of preemptive application QX-314, which induces long-lasting analgesia, on the development of trigeminal neuropathic pain following nerve injury.
Voltage-gated sodium channels (Navs) in the trigeminal ganglion play a critical role in modulating nociceptive sensory signaling. Tissue and nerve damage alter the expression and function of specific α-subunits of Navs, which can change the excitability of sensory neurons resulting in chronic pain conditions [1314]. However, there is no evidence for a role of Navs in preemptive analgesia-induced antinociception.
Herein, we investigated the effects of preemptive analgesia on the development of trigeminal neuropathic pain. For this purpose, mechanical allodynia was evaluated in rats with chronic constriction injury of the infraorbital nerve (CCI-ION) after preemptive perineural application of 2% QX-314 to the infraorbital nerve. Additionally, we examined changes in the expression of glial fibrillary acidic protein (GFAP) and phosphorylated-p38 (p-p38) in the trigeminal ganglion in these rats following CCI-ION, to investigate the mechanisms underlying the anti-allodynic effects of preemptive analgesia. We also investigated the role of Navs in the antinociception produced by preemptive application of QX-314 by analyzing the changes in the expression of Nav isoforms in the trigeminal ganglion.
Experiments were performed using male Sprague-Dawley rats, weighing between 230 and 260 g. All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the School of Dentistry, Kyungpook National University (approved No. 2015-0053) and were carried out in accordance with the ethical guidelines for the investigation of experimental pain in conscious animals of the International Association for the Study of Pain. Animals were maintained in a temperature-controlled room (23±1℃) with a 12-/12-h light-dark cycle. Food and water were freely available. All experimental procedures were performed in a blind fashion.
Surgical procedures were performed under ketamine (40 mg/kg) and xylazine (4 mg/kg) anesthesia. Under anesthesia, CCI-ION was performed based on the original description [15] with modifications [16]. A 1cm-long incision was made along the gingivo-buccal margin. The incision was begun proximal to the first molar. Approximately 0.5 cm of the infraorbital nerve was separated from the adhering tissue and 2 ligatures (5-0 chromic gut) were tied loosely around the nerve. The incision was sutured at 2 points, using 4-0 silk. The sham operation was performed without ligation of the infraorbital nerve.
Rats were tested 3 days before the CCI-ION and at 1, 3, 7, 10, 14, 17, 21, 24, 30, and 40 days after the surgery. All behavioral tests were conducted between 0700 and 1800 hours. The withdrawal behavioral responses produced by 10 successive trials of a ramp of air-puff pressure (4 sec of duration, 10 sec of interval) were examined as described previously [17181920]. The intensity of the air-puff pressure was controlled by a pneumatic pump module (BH2 System; Harvard Apparatus, MA). Air puffs were applied through a 26-gauge metal tube (length, 10 cm) located 1 cm from the skin at a 90° angle. The evoked receptive field of air-puff stimuli was limited because a 26-gauge metal tube was used for application. The air-puff threshold was determined as the air-puff pressure at which each rat responded in 50% of the trials. The cut-off pressure for the air puff was 40 psi, as described previously [21222324]. The naïve rats did not respond to a pressure of less than 40 psi. A significant decrease in the threshold for an air-puff response, compared with the pre-operative values, was defined as mechanical allodynia.
Seven days after CCI-ION, anesthetized rats (n=4 per group) were perfused through the ascending aorta with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The trigeminal ganglion was then dissected out, postfixed in the same fixative at 4℃ overnight, and then placed in 30% sucrose in 0.1 M phosphate buffer overnight. Transverse frozen sections (free-floating, 20 µm thickness) were obtained using a cryostat and processed for immunofluorescence. All sections were blocked with 5% goat serum in phosphate-buffered saline containing 0.2% Triton X-100 for 1 h at room temperature and then incubated overnight at 4℃ with primary antibodies for GFAP (satellite cell marker, 1:4000; CST, Boston, MA), and p-p38 MAPK (1:200; CST), and activating transcription factor-3 (ATF-3, neuronal injury marker, 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). Following incubation, tissue sections were washed and incubated for 2 h at room temperature with an Alexa 555-conjugated rabbit IgG antibody (1:200; Invitrogen, Carlsbad, CA).
For double immunofluorescence, the sections were incubated with a primary antibody mixture of p-p38 MAPK and NeuN (neuronal marker, 1:5000; Millipore, Temecula, CA) or GFAP (1:4000; CST) overnight at 4℃, followed by a mixture of Alexa 555-conjugated rabbit IgG and Alexa 488-conjugated mouse IgG (1:200; Invitrogen). The stained sections were then observed under a fluorescence microscope (BX 631 and U-RFL-T; Olympus, Japan). Co-localization analysis of immunofluorescence images was performed using a confocal laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany).
Twelves sections (three sections from each trigeminal ganglion) were examined in each group using a fluorescence microscope to quantify ATF3-immunoreactivity. The number of ATF3-immunoreactive neurons was counted at 400X magnification, using a counting grid. To quantify the GFAP and phosphorylated p38-positive cells in the trigeminal ganglion, images were captured from the trigeminal ganglion in each condition: sham, CCI-ION, and QX-314 + CCI-ION. The threshold level for defining cells as immunopositive was set at 15 grey levels in images with 0-255 gray levels. The images were captured at 20X magnification in the ganglion area using Image J software (NIH, Bethesda, MD) [25].
Rats (n=6 per group) were sacrificed on postoperative day (POD) 7 by decapitation. The trigeminal ganglion was immediately removed from each animal and quickly frozen in liquid nitrogen. Samples were sonicated with Biorupture (Cosmo Bio., Tokyo, Japan) in a lysis buffer containing protease and a phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). For western blotting, total proteins (40 mg) were separated in a 4%-12% gradient NuPAGE Novex Bis-Tris gel (Invitrogen) and transferred onto a nitrocellulose membrane. The membranes were then blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 for 1 hour at room temperature, followed by incubation with primary antibodies to Nav1.3, 1.7, 1.8, and 1.9 (1:400, Alomone Labs, Jerusalem, Israel) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:10000; Santa Cruz) at 4℃ overnight. The blots were subsequently incubated with secondary antibody horseradish peroxidase for 1 hour at room temperature. The intensity of each band was determined by quantitative chemiluminescence using an Amersham Imager 600 (GE Healthcare, Buckinghamshire, UK). Image J software (NIH) was used to quantify specific bands.
To confirm inflammation of the infraorbital nerve induced by the CCI-ION, we measured the extravasated concentration of Evans blue dye bound to the plasma protein, as described previously [2627]. At the conclusion of each experiment, the animals were anesthetized with ketamine (40 mg/kg) and xylazine (4 mg/kg). Evans blue dye (1% solution, 5 ml/kg) was injected into the right femoral vein. For 15 min following the injection of the dye, each rat was perfused with heparinized normal saline. The infraorbital nerve was dissected, weighed, and stored at −20℃ until the analysis was performed. The tissues were incubated overnight in a 7:3 mixture of acetone and 0.5% sodium sulfate solution at room temperature with intermittent shaking. After incubation, the samples were centrifuged at 300 rpm for 10 min and the supernatant was separated. The amount of dye present in each sample was analyzed by spectrophotometric measurement of the absorbance at 620 nm. The recovery of the extravasated dye per gram weight of tissue (mg/g) was calculated by comparing the absorbance of the supernatant with a standard curve. The standard curve was generated from a series of the same extraction solution mixed with known amounts of Evans blue dye.
Single perineural application of 2% QX-314 (10 ml) to the injured area of the infraorbital nerve was performed immediately after the CCI-ION operation. Changes in air-puff thresholds were then measured. We also evaluated mechanical allodynia following the combined application of QX-314 with capsaicin (1 mg/10 ml) to the infraorbital nerve in rats following CCI-ION.
To examine the effects of double application of QX-314 on the development of trigeminal neuropathic pain, perineural application of 2% QX-314 to the injured area of the infraorbital nerve was performed again 12 h after the first application of QX-314. Air-puff thresholds were then measured.
To investigate whether QX-314-induced prolonged analgesia attenuates the development of neuropathic mechanical allodynia when pain has been already established, we performed single and double applications of 2% QX-314 on POD 7. Changes in air-puff thresholds were then examined.
Immediate double application of QX-314 produced significant anti-allodynic effects in rats following CCI-ION. To investigate the mechanisms involved, we examined whether QX-314-induced preemptive analgesia affects the infraorbital nerve inflammation produced by CCI-ION. The concentration of extravasated Evans blue dye was measured on POD 7 in rats that had received immediate double application of QX-314. We examined the changes in the expression of ATF3, a neuronal injury marker, in the trigeminal ganglion on POD 7 in the QX-314-treated rats. Changes in the expression of GFAP and p-p38 in the trigeminal ganglion were also investigated on POD 7 in rats who had received double applications of 2% QX-314.
To investigate a role of Navs in the antinociceptive effects of QX-314-induced preemptive analgesia, we analyzed the expression of Nav1.3, 1.7, 1.8, and 1.9 in the trigeminal ganglion on POD 7 by western blot analysis in rats following CCI-ION after immediate double administration of 2% QX-314.
QX-314 and capsaicin were purchased from Sigma (St Louis, MO). Capsaicin stock solution was prepared in 100% ethanol and diluted to the working concentration with 5% Tween 20 in sterile saline immediately before use. The final working concentration of ethanol was 3%. QX-314 was prepared in sterile saline.
The differences between behavioral data were compared using repeated measures ANOVA followed by Holm-Sidak post hoc analysis. The western blotting data were analyzed by one-way analysis of variance followed by Holm-Sidak post hoc analysis. In all statistical comparisons, p<0.05 was considered statistically significant. All data are presented as the mean±SEM.
We found that preemptive application of QX-314 attenuated neuropathic mechanical allodynia in rats following CCI-ION (Fig. 1). CCI-ION produced severe mechanical allodynia that appeared on POD 1. Maximal CCI-ION-induced mechanical allodynia were observed on POD 7 and was maintained until at least POD 30. Vehicle treatment did not affect the mechanical allodynia produced by CCI-ION. However, a single application of 2% QX-314 immediately after nerve injury significantly attenuated the neuropathic mechanical allodynia (F(3,24)=30.300, p<0.05). Treatment with QX-314 significant boosted early recovery from mechanical allodynia to the basal level compared with the mechanical allodynia observed following treatment with vehicle alone. Combined treatment of 2% QX-314 with capsaicin (1 mg) showed similar anti-allodynic effects to those observed following treatment with 2% QX-314 alone. Therefore, subsequent experiments were performed using QX-314 without capsaicin.
Fig. 2 illustrates the effects of double application of 2% QX-314 on neuropathic mechanical allodynia. Immediate double application of QX-314 significantly attenuated mechanical allodynia and boosted early recovery from mechanical allodynia to the basal level compared with the responses observed following a single application of QX-314 (F(2,18)=81.231, p<0.05). Double application of QX-314 dramatically alleviated mechanical allodynia in the 10 days following injury of the infraorbital nerve.
To investigate the effects of treatment with QX-314 when pain is already established, 2% QX-314 was applied on POD 7. Neither single nor double application of 2% QX-314 affected the CCI-ION-induced mechanical allodynia (Fig. 3).
To investigate the mechanisms underlying the preemptive analgesia-induced anti-allodynic effects of QX-314, we measured the changes in the concentration of Evans blue dye extravasated from the infraorbital nerve after immediate double application of 2% QX-314 (Fig. 4). The group that underwent CCI-ION showed a significant increase in the extravasated Evans blue dye concentration compared with the results in the sham group (132.3±15.1 mg, p<0.05). However, double application of 2% QX-314 did not affect the extravasated Evans blue dye concentration compared with concentration observed in the vehicle group. We also investigated whether immediate double application of 2% QX-314 blocked the infraorbital nerve injury by analyzing changes in the level of ATF-3, a neuronal injury marker, in the trigeminal ganglion. CCI-ION significantly increased ATF-3 immunoreactivity (5.6±0.7 cells/section) in the trigeminal ganglion. However, double application of 2% QX-314 did not alter the number of cells with ATF-3 immunoreactivity (6.0±0.3 cells/section) in rats following CCI-ION (Fig. 5).
We also examined the expression of GFAP and p-p38 in the trigeminal ganglion after injury to the infraorbital nerve on POD 7. CCI-ION upregulated the GFAP and p-p38 expression in the trigeminal ganglion. P-p38 labeling varies depending on the size of neuron (17% in small sized neurons; 50% in medium sized neurons; 33% in large sized neurons). Immediate double application of 2% QX-314 reduced the upregulation of GFAP and p-p38 expression in the trigeminal ganglion following CCI-ION. The upregulations of the area density of GFAP and p-p38 immunoreactivity were significantly decreased following treatment with QX-314, respectively (Fig. 6). The increased p-p38 expression was co-localized with NeuN, a neuronal marker, but not with GFAP, a satellite glial cell marker (Fig. 7).
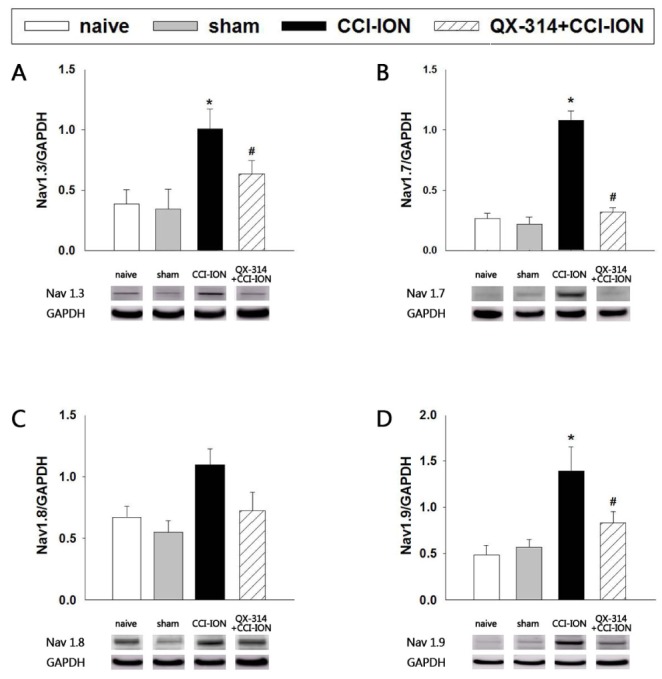
Fig. 8 illustrates the effects of preemptive application of QX-314 on the expression of Nav1.3, 1.7, 1.8, and 1.9 in the trigeminal ganglion. CCI-ION significantly increased the expression of Nav1.3, 1.7, and 1.9 (p<0.05) but did not affect the expression of Nav1.8 (P=0.071) compared with the expression observed in the sham group. The sham operation did not affect the expression of Nav isoforms. Immediate double application of 2% QX-314 significantly inhibited the up-regulation of the expression of Nav1.3, 1.7, and 1.9 observed following CCI-ION (p<0.05) but did not inhibit the CCI-ION-induced upregulation of Nav1.8 (p=0.355).
The present study demonstrates that preemptive application of QX-314 reduced neuropathic mechanical allodynia in rats following CCI-ION through the inhibition of Nav isoform expression in the trigeminal ganglion. Immediate application of 2% QX-314 to the injured area of the infraorbital nerve significantly reduced neuropathic mechanical allodynia. Although preemptive application of QX-314 did not affect nerve injury-induced inflammation or ATF-3 expression in the trigeminal ganglion, it did attenuate the upregulation of GFAP and p-p38 expression in the trigeminal ganglion following CCI-ION. In addition, preemptive application of QX-314 significantly reduced the upregulated expression of Nav1.3, 1.7, and 1.9 induced by CCI-ION. These results provide a potential preemptive therapeutic strategy for the treatment of neuropathic pain following nerve injury.
Preemptive analgesia may decrease post-operative pain in the clinic. In previous clinical studies, preemptive administration of an analgesic agent has been shown to improve postoperative pain control in case of laparoscopic radical prostatectomy [28], thoracotomy [29], and third molar extraction [3031]. Additionally, preemptive application of 1% lidocaine for 10 minutes prior to L4 and L5 bilateral dorsal rhizotomy significantly attenuates hindlimb mechanical allodynia in patients with failed back surgery syndrome [32]. In animal studies, pretreatment with intravenous ketamine inhibits inflammatory pain behavior and c-fos expression following formalin injection in rats as a preemptive analgesia [33] and intraperitoneal administration of diclofenac has preemptive analgesic effects on acute thermal pain and formalin-induced inflammatory pain in rats [34]. These results suggest that pretreatment with analgesics plays an important role in preemptive analgesia. In contrast to the beneficial role of preemptive analgesia, no beneficial effects of preemptive analgesia have been reported in postoperative pain control. Thoracic extradural block with bupivacaine does not produce an early preemptive effect following thoracotomy [35]. In an animal model, preincisional treatment is not more beneficial than postincisional treatment for postoperative pain relief [36]. The lack of effects of preemptive analgesia in these cases may be caused by central sensitization which is responsible for the secondary development of pain, including neuronal plasticity [37].
Our findings demonstrate that preemptive application of QX-314 inhibited neuropathic mechanical allodynia in rats following CCI-ION. These results suggest that preemptive or early pain control is a beneficial strategy for the treatment of neuropathic pain following nerve injury. QX-314 was selected to be used as a preemptive analgesic in this study because it produces long-lasting analgesia via co-administration with capsaicin, a TRPV1 agonist [8910]. The rationale behind this combined treatment method is that the activation of TRPV1, which is peripherally expressed in primary afferent nociceptors [3839], by capsaicin allows QX-314 to enter neurons. However, we found that the anti-allodynic effects produced by the combined treatment of 2% QX-314 with capsaicin were not different from those of QX-314 alone. This suggests that, under our experimental conditions, the application of QX-314 alone serves as a preemptive analgesic agent without capsaicin. Therefore, we used QX-314 alone as a preemptive analgesic agent for all of our subsequent experiments. We also carried out experiments blocking the transmission of nerve signaling through the application of lidocaine, as an alternative preemptive analgesics. However, preemptive application of lidocaine did not affect the development of trigeminal neuropathic pain. Preemptive application of QX-314, which has long lasting sensory blocking, produced a significant inhibition of the development of trigeminal neuropathic pain. Thus, our data suggest that long-lasting blockade of pain transmission immediately following nerve injury produces significant attenuation of the development of trigeminal neuropathic pain. Our present study is the first to demonstrate the usefulness of QX-314 as a preemptive analgesic agent in neuropathic pain control. The double application of 2% QX-314 dramatically reduced nerve injury-induced allodynia compared with the responses observed following a single application of 2% QX-314. Moreover, a single or double application of 2% QX-314 on POD 7, when pain is already established, did not affect neuropathic mechanical allodynia. These results suggest that QX-314-induced analgesia attenuates the development of neuropathic pain only when pain has not been established.
Satellite glial cells, the main type of glial cell in the sensory ganglia, respond to damage or inflammation of a sensory neuron by proliferating and expressing GFAP, similar to the glial responses observed in the spinal cord [40]. Nerve injury or inflammation leads to proliferation and hypertrophy of satellite glial cells. In these conditions, satellite glial cells upregulate various molecules, including neurotrophins, transforming growth factor-α, tumor necrosis factor-α, isolectin-B4, and functional gap junctions [40]. Our current data indicate that, following CCI-ION, GFAP expression is upregulated and that this upregulation is inhibited by QX-314-induced preemptive analgesia in the trigeminal ganglion. The participation of satellite cells in nerve injury or damage in the orofacial area has previously been reported. The expression of GFAP in satellite glial cells increases after axotomy of the infraorbital nerve [41], CCI-ION [42], and peripheral tooth injury [43]. These results, together with our present data, suggest that modulation of satellite cell activity in the trigeminal ganglion plays an important role in the attenuation of neuropathic pain in QX-314-induced preemptive analgesia. However, the underlying mechanism through which satellite cells are involved in preemptive analgesia should be investigated in through further study.
Our current results also showed that p-p38 expression was upregulated in the trigeminal ganglion following CCI-ION. In addition, the p-p38 immunoreactivity was localized to neurons in the trigeminal ganglion. In previous reports, p-p38 immunoreactivity has been shown to be elevated in dorsal root ganglion nociceptor neurons following spinal nerve ligation [4445] and chronic constriction injury of the sciatic nerve [46]. Treatment with a p38 inhibitor has been shown to reduce neuropathic mechanical allodynia [4445]. These results, together with our present findings, suggest that the upregulation of neuronal p-p38 participates in the development of neuropathic pain following CCI-ION.
The present study demonstrate that nerve injury increased GFAP expression in satellite cells and p-p38 expression in neuronal cells in the trigeminal ganglion. Preemptive application of QX-314 attenuated both the upregulation of GFAP expression in satellite cells and the upregulation of p-p38 expression in neuronal cells. The possible interrelationship between satellite glia and neuronal cells in the trigeminal ganglion has been already reported. Activation of satellite glial cells in the trigeminal ganglion leads to the hyper-activation of neurons via IL-1β, resulting in ectopic tooth pulp pain following tooth inflammation [47]. Moreover, functional interactions between neurons and satellite glial cells or neurons and neurons in the trigeminal ganglion are involved in the orofacial extraterritorial pain associated with trigeminal nerve injury [48]. These results suggest that a functional interaction between neurons and glial cells is critical in the persistent orofacial pain associated with orofacial inflammation and trigeminal nerve injury [49]. However the present data cannot exclude a possible underlying mechanisms that are produced by events independent of these two factors. Therefore, in order to fully elucidate the underlying cellular mechanisms involved, future studies are required.
Interestingly, a recent study reported that complete Freund's Adjuvant (CFA)-induced inflammatory pain increased the co-expression of CXCR5 and Nav1.8 in DRG neurons [50]. This co-expression was shown to be reduced by intrathecal administration of a p38 inhibitor [50]. Furthermore, TNF-α-mediated nociceptive hyperexcitability has been found to increase the expression of p38 dependent tetrodotoxin-resistant (TTX-r) sodium channels [51]. Modulation of p38-dependent TTX-r sodium channels may lead to the development of effective treatments for inflammatory pain [51]. The upregulation of p-p38 expression in neurons of the trigeminal ganglion should, therefore, be investigated to determine whether voltage-gated sodium channels participate in the anti-nociceptive effects of preemptive analgesia in rats following CCI-ION.
Navs, which are large transmembrane protein complexes composed of 9 different pore-forming α-subunits (Nav 1.1-Nav 1.9) and auxiliary β-subunits [52], exhibit distinct expression patterns and electrophysiological and pharmacological properties [53]. The findings obtained in our rat model of trigeminal neuropathic pain indicate that CCI-ION upregulated the expression of Nav1.3, 1.7, and 1.9 in the trigeminal ganglion. Navs in sensory neurons participate in the development of neuropathic pain in animal and clinical studies. Nav1.3 is upregulated in the peripheral and central nervous systems of rats following nerve injury, while its knockdown attenuates nerve injury-induced mechanical allodynia in the spared nerve injury model [54]. Nav1.7 is upregulated in the DRG of rats with painful diabetic neuropathy [55] and with chronic constriction injury of the sciatic nerve [56]. In addition, chronic constriction injury of the inferior alveolar nerve increases Nav1.7 expression in the trigeminal ganglion and subcutaneous injection of botulinum type A produces anti-allodynic effects through attenuation of upregulated Nav1.7 expression [24]. Moreover, Nav1.9 expression increases in the DRG after lumbar disc injury [57]. A previous clinical study demonstrated that missense mutations in Nav1.9 may be a cause of painful peripheral neuropathy [55]. These results suggest that modulation of the α-subunits of Nav in sensory neurons may be a viable strategy for the treatment of chronic pain conditions, including neuropathic pain [131453]. Our present analysis demonstrates that p-p38 expression is observed in neuronal cells of varied sizes in the trigeminal ganglion following nerve injury. Western blot analysis revealed that nerve injury increased the expression of Nav isoforms in the trigeminal ganglion. Preemptive application of QX-314 attenuated trigeminal mechanical allodynia as well as the upregulated expression of Nav isoforms following CCI-ION. These results suggest that the up-regulated expression of p-p38 and Nav isoforms participates in the development of trigeminal neuropathic pain following nerve injury. Moreover, our present analysis demonstrates that preemptive application of QX-314 significantly inhibited the upregulation of Nav1.3, 1.7, and 1.9 expressions in the trigeminal ganglion in rats following CCI-ION. These results suggest that early or preemptive blockade of pain signals in the injured nerve area may inhibit the expression of all Nav isoforms in the trigeminal ganglion, thus playing an important role in the development of neuropathic pain in sensory neurons following nerve injury. In addition, long-lasting preemptive analgesia provides a powerful treatment strategy for preventing the development of neuropathic pain following nerve injury through the attenuation of upregulated Nav expression in the trigeminal ganglion. However, in order to fully differentiate the type of neurons that express p-p38, or the Navs isoforms, further studies will be needed.
We found, from our current analysis, that QX-314-induced preemptive analgesia did not alter the extravasated Evans blue dye concentration following CCI-ION. Furthermore, preemptive analgesia did not reduce the number of cell expressing ATF3, a neuronal injury marker. These results suggest that QX-314-induced preemptive analgesia does not seem to be mediated by the blockage of inflammation or nerve injury following CCI-ION.
In conclusion, QX-314-induced preemptive analgesia reduces the neuropathic mechanical allodynia and the upregulation of GFAP and p-p38 expression in the trigeminal ganglion in rats following CCI-ION. Moreover, QX-314 induces preemptive analgesia, significantly reducing upregulated expression of Nav1.3, 1.7, and 1.9 produced by CCI-ION. These results suggest that long-lasting preemptive analgesia inhibits neuropathic pain through the regulation of Nav isoform expression in the trigeminal ganglion. Importantly, these results provide a potential preemptive therapeutic strategy for the treatment of neuropathic pain following nerve injury.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2017R1A5A2015391).
Notes
Author contributions: J.H.Y., J.Y.S. and D.K.A. contributed to conception, design and data analysis, draft and critically revised the manuscript. M.J.K., S.H.K. and J.S.J. contributed to data analysis. Y.C.B. contributed critical revised manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
References
1. Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996; 12:50–55. PMID: 8722735.

2. Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. 1999; 43:563–567. PMID: 10342006.

3. Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006; 7:626–634. PMID: 16942948.

4. Subramaniam B, Pawar DK, Kashyap L. Pre-emptive analgesia with epidural morphine or morphine and bupivacaine. Anaesth Intensive Care. 2000; 28:392–398. PMID: 10969365.

5. Yegin A, Erdogan A, Kayacan N, Karsli B. Early postoperative pain management after thoracic surgery; pre- and postoperative versus postoperative epidural analgesia: a randomised study. Eur J Cardiothorac Surg. 2003; 24:420–424. PMID: 12965314.

6. Troncy E, Junot S, Keroack S, Sammut V, Pibarot P, Genevois JP, Cuvelliez S. Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997-1999). J Am Vet Med Assoc. 2002; 221:666–672. PMID: 12216906.

7. Batista LM, Batista IM, Almeida JP, Carvalho CH, Castro-Costa SB, Castro-Costa CM. Preemptive analgesic effect of lidocaine in a chronic neuropathic pain model. Arq Neuropsiquiatr. 2009; 67:1088–1092. PMID: 20069225.

8. Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007; 449:607–610. PMID: 17914397.

9. Kim HY, Kim K, Li HY, Chung G, Park CK, Kim JS, Jung SJ, Lee MK, Ahn DK, Hwang SJ, Kang Y, Binshtok AM, Bean BP, Woolf CJ, Oh SB. Selectively targeting pain in the trigeminal system. Pain. 2010; 150:29–40. PMID: 20236764.

10. Sagie I, Kohane DS. Prolonged sensory-selective nerve blockade. Proc Natl Acad Sci U S A. 2010; 107:3740–3745. PMID: 20133669.

11. Gerner P, Binshtok AM, Wang CF, Hevelone ND, Bean BP, Woolf CJ, Wang GK. Capsaicin combined with local anesthetics preferentially prolongs sensory/nociceptive block in rat sciatic nerve. Anesthesiology. 2008; 109:872–878. PMID: 18946300.

12. Ries CR, Pillai R, Chung CC, Wang JT, MacLeod BA, Schwarz SK. QX-314 produces long-lasting local anesthesia modulated by transient receptor potential vanilloid receptors in mice. Anesthesiology. 2009; 111:122–126. PMID: 19512885.

13. Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001; 22:27–31. PMID: 11165669.
14. Dib-Hajj SD, Black JA, Waxman SG. Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 2009; 10:1260–1269. PMID: 19818036.

15. Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997; 116:97–103. PMID: 9305818.

16. Lim EJ, Jeon HJ, Yang GY, Lee MK, Ju JS, Han SR, Ahn DK. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31:1322–1329. PMID: 17618720.

17. Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009; 146:114–120. PMID: 19665300.

18. Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain. 2009; 13:568–575. PMID: 18774318.

19. Kim MJ, Park YH, Yang KY, Ju JS, Bae YC, Han SK, Ahn DK. Participation of central GABAA receptors in the trigeminal processing of mechanical allodynia in rats. Korean J Physiol Pharmacol. 2017; 21:65–74. PMID: 28066142.
20. Kim MJ, Shin HJ, Won KA, Yang KY, Ju JS, Park YY, Park JS, Bae YC, Ahn DK. Progesterone produces antinociceptive and neuroprotective effects in rats with microinjected lysophosphatidic acid in the trigeminal nerve root. Mol Pain. 2012; 8:16. PMID: 22429647.

21. Han SR, Yang GY, Ahn MH, Kim MJ, Ju JS, Bae YC, Ahn DK. Blockade of microglial activation reduces mechanical allodynia in rats with compression of the trigeminal ganglion. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 36:52–59. PMID: 22019843.

22. Jeon HJ, Han SR, Lim KH, Won KA, Bae YC, Ahn DK. Intracisternal administration of NR2 subunit antagonists attenuates the nociceptive behavior and p-p38 MAPK expression produced by compression of the trigeminal nerve root. Mol Pain. 2011; 7:46. PMID: 21651766.

23. Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 38:149–158. PMID: 22449477.

24. Yang KY, Kim MJ, Ju JS, Park SK, Lee CG, Kim ST, Bae YC, Ahn DK. Antinociceptive effects of botulinum toxin type A on trigeminal neuropathic pain. J Dent Res. 2016; 95:1183–1190. PMID: 27418174.

25. Cho YS, Ryu CH, Won JH, Vang H, Oh SB, Ro JY, Bae YC. Rat odontoblasts may use glutamate to signal dentin injury. Neuroscience. 2016; 335:54–63. PMID: 27555550.

26. Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1betainduced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005; 117:204–213. PMID: 16098663.
27. Harada M, Takeuchi M, Fukao T, Katagiri K. A simple method for the quantitative extraction of dye extravasated into the skin. J Pharm Pharmacol. 1971; 23:218–219. PMID: 4396896.

28. Trabulsi EJ, Patel J, Viscusi ER, Gomella LG, Lallas CD. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology. 2010; 76:1122–1124. PMID: 20570321.

29. Ryu HG, Lee CJ, Kim YT, Bahk JH. Preemptive low-dose epidural ketamine for preventing chronic postthoracotomy pain: a prospective, double-blinded, randomized, clinical trial. Clin J Pain. 2011; 27:304–308. PMID: 21178605.
30. Pandit MK, Godhi S, Lall AB. Preoperative intravenous tramadol versus diclofenac for preventing postoperative pain after third molar surgery: a comparative study. J Maxillofac Oral Surg. 2011; 10:306–309. PMID: 23204745.

31. Shah R, Mahajan A, Shah N, Dadhania AP. Preemptive analgesia in third molar impaction surgery. Natl J Maxillofac Surg. 2012; 3:144–147. PMID: 23833488.

32. Rooney BA, Crown ED, Hulsebosch CE, McAdoo DJ. Preemptive analgesia with lidocaine prevents Failed Back Surgery Syndrome. Exp Neurol. 2007; 204:589–596. PMID: 17261281.

33. Lee IH, Lee IO. Preemptive effect of intravenous ketamine in the rat: concordance between pain behavior and spinal fos-like immunoreactivity. Acta Anaesthesiol Scan. 2005; 49:160–165.

34. Hasani AS, Soljakova M, Jakupi MH, Ustalar-Ozgen SZ. Preemptive analgesic effect of diclofenac: experimental study in rats. Middle East J Anaesthesiol. 2011; 21:355–360. PMID: 22428489.
35. Aguilar JL, Rincón R, Domingo V, Espachs P, Preciado MJ, Vidal F. Absence of an early pre-emptive effect after thoracic extradural bupivacaine in thoracic surgery. Br J Anaesth. 1996; 76:72–76. PMID: 8672384.

36. Chang WK, Tao YX, Chuang CC, Chen PT, Chan KH, Chu YC. Lack of beneficial effect for preemptive analgesia in postoperative pain control: verifying the efficacy of preemptive analgesia with N-methyl-D-aspartate receptor antagonists in a modified animal model of postoperative pain. Anesth Analg. 2011; 112:710–718. PMID: 21233503.
37. Seetharaman SV, Prudencio M, Karch C, Holloway SP, Borchelt DR, Hart PJ. Immature copper-zinc superoxide dismutase and familial amyotrophic lateral sclerosis. Exp Biol Med (Maywood). 2009; 234:1140–1154. PMID: 19596823.

38. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.

39. Jhaveri MD, Elmes SJ, Kendall DA, Chapman V. Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naïve, carrageenan-inflamed and neuropathic rats. Eur J Neurosci. 2005; 22:361–370. PMID: 16045489.

40. Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005; 48:457–476. PMID: 15914252.

41. Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004; 110:290–298. PMID: 15275779.

42. Donegan M, Kernisant M, Cua C, Jasmin L, Ohara PT. Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve. Glia. 2013; 61:2000–2008. PMID: 24123473.

43. Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol. 1995; 131:11–22. PMID: 7895805.

44. Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003; 23:4017–4022. PMID: 12764087.

45. Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003; 23:2517–2521. PMID: 12684435.
46. Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004; 20:2881–2895. PMID: 15579142.

47. Komiya H, Shimizu K, Noma N, Tsuboi Y, Honda K, Kanno K, Ohara K, Shinoda M, Ogiso B, Iwata K. Role of neuron-glial interaction mediated by IL-1β in ectopic tooth pain. J Dent Res. 2018; 97:467–475. PMID: 29131694.

48. Kobayashi A, Shinoda M, Sessle BJ, Honda K, Imamura Y, Hitomi S, Tsuboi Y, Okada-Ogawa A, Iwata K. Mechanism involed in extraterritorial facial pain following cervical spinal nerve injury in rats. Mol Pain. 2011; 7:12. PMID: 21310020.
49. Iwata K, Katagiri A, Shinoda M. Neuron-glia interaction is a key mechanism underlying persistent orofacial pain. J Oral Sci. 2017; 59:173–175. PMID: 28637974.

50. Wu XB, Cao DL, Zhang X, Jiang BC, Zhao LX, Qian B, Gao YJ. CXCL13/CXCR5 enhances sodium channel Nav1.8 current density via p38 MAP kinase in primary sensory neurons following inflammatory pain. Sci Rep. 2016; 6:34836. PMID: 27708397.

51. Gudes S, Barkai O, Caspi Y, Katz B, Lev S, Binshtok AM. The role of slow and persistent TTX-resistant sodium currents in acute tumor necrosis factor-α-mediated increase in nociceptors excitability. J Neurophysiol. 2015; 113:601–619. PMID: 25355965.

52. Marban E, Yamagishi T, Tomaselli GF. Structure and function of voltage-gated sodium channels. J Physiol. 1998; 508:647–657. PMID: 9518722.

53. Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005; 57:397–409. PMID: 16382098.

54. Samad OA, Tan AM, Cheng X, Foster E, Dib-Hajj SD, Waxman SG. Virus-mediated shRNA knockdown of Na(v)1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol Ther. 2013; 21:49–56. PMID: 22910296.

55. Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JG, Gerrits MM, Tyrrell L, Lauria G, Faber CG, Dib-Hajj SD, Merkies IS, Waxman SG. PROPANE Study Group. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain. 2014; 137:1627–1642. PMID: 24776970.

56. Liu C, Cao J, Ren X, Zang W. Nav1.7 protein and mRNA expression in the dorsal root ganglia of rats with chronic neuropathic pain. Neural Regen Res. 2012; 7:1540–1544. PMID: 25657691.
57. Watanabe K, Larsson K, Rydevik B, Konno S, Nordborg C, Olmarker K. Increase of sodium channels (nav 1.8 and nav 1.9) in rat dorsal root ganglion neurons exposed to autologous nucleus pulposus. Open Orthop J. 2014; 8:69–73. PMID: 24843387.
Fig. 1
The effects of a single application of 2% QX-314 on neuropathic mechanical allodynia in rats with chronic constriction injury of the infraorbital nerve (CCI-ION).
CCI-ION produced significantly mechanical allodynia. Treatment with vehicle did not alter the CCI-ION-induced mechanical allodynia. Perineural application of QX-314 immediately following CCI-ION significantly reduced neuropathic mechanical allodynia. Combined treatment with capsaicin (1 mg) produced attenuation of neuropathic mechanical allodynia. However, the anti-allodynic effects following treatment with QX-314 in combination with capsaicin did not differ from these effects following treatment with QX-314 alone. Arrow, single application of QX-314. There were 8 animals in each group. *p<0.05, vehicle- vs. QX-314- or cap+QX-314-treated group.
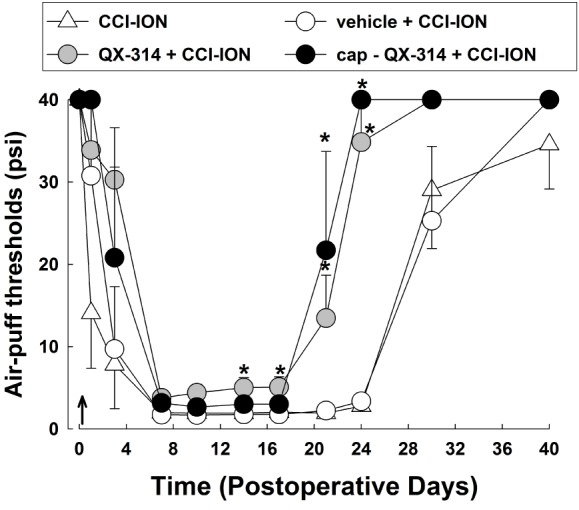
Fig. 2
The effects of immediate double application of 2% QX-314 on CCI-ION-induced mechanical allodynia.
Perineural double application of QX-314 immediately following CCI-ION significantly attenuated neuropathic mechanical allodynia compared with the effects of a single application of QX-314. Arrow, double application of QX-314. There were 8 animals in each group. *p<0.05, vehicle- vs. QX-314 (single)-treated group. #p<0.05, QX-314 (single)- vs. QX-314 (double)-treated group.
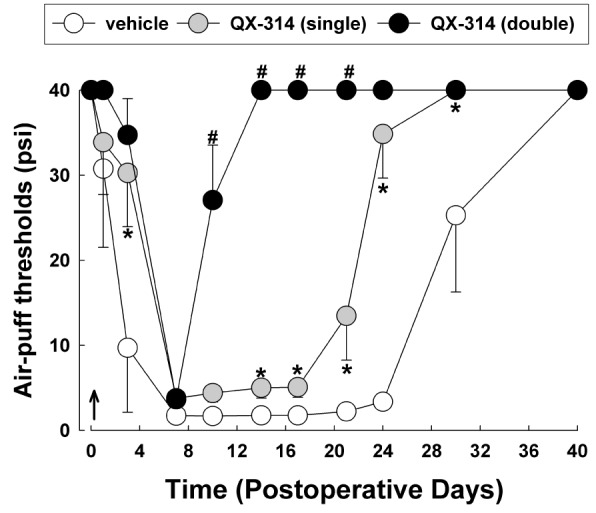
Fig. 3
The effects of 2% QX-314 on CCI-ION-induced mechanical allodynia on POD 7.
Single or double application of QX-314 did not affect neuropathic mechanical allodynia when pain was already established. Arrow, application of QX-314 on POD 7. There were 8 animals in each group.
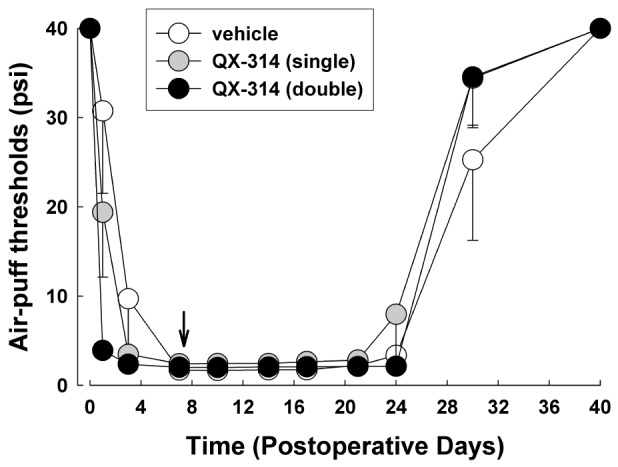
Fig. 4
The effects of immediate double application of 2% QX-314 on the concentration of extravasated Evans blue dye in rats following CCI-ION.
CCI-ION produced a significant increase in the extravasated Evans blue dye concentration. However, QX-314-induced preemptive analgesia did not affect the extravasated Evans blue dye concentration. *p<0.05, sham vs. vehicle+CCI-ION group. There were 6 animals in each group.
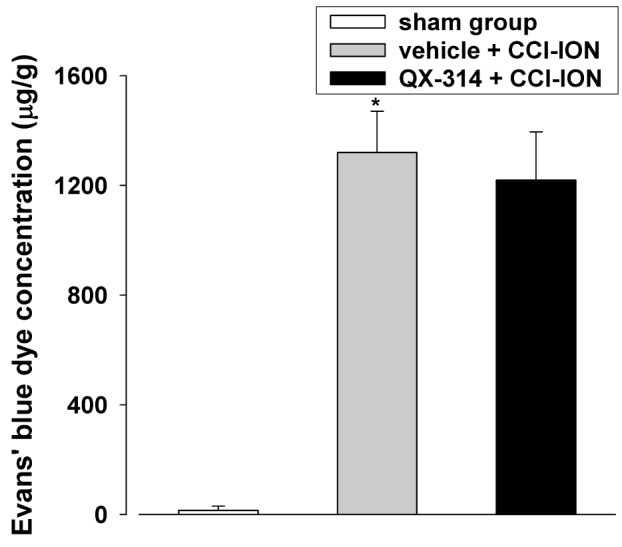
Fig. 5
The effect of immediate double application of 2% QX-314 on the expression of ATF-3, a neuronal injury marker, in rats following CCI-ION.
CCI-ION significantly increased the number of ATF-3-immunoreactive cells in the trigeminal ganglion. However, application of QX-314 did not affect the number of cells with ATF-3 immunoreactivity following CCI-ION. Scale bar, 200 µm.
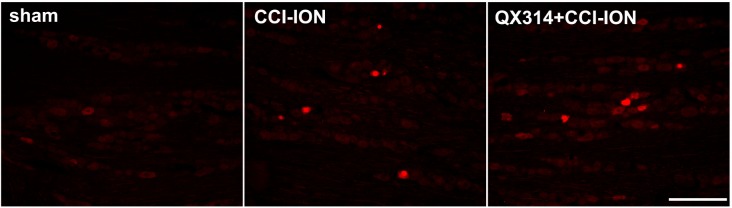
Fig. 6
The effects of immediate double application of 2% QX-314 on GFAP and p-p38 expression in the trigeminal ganglion.
(A) CCI-ION upregulated GFAP and p-p38 expression on POD 7. Double application of QX-314 reduced the GFAP and p-p38 upregulation in the trigeminal ganglion following CCI-ION. Scale bar, 200 µm. (B, C) CCI-ION increases the area density of GFAP and p-p38 immunoreactivity compared to the immunoreactivity observed in the sham group. Treatment with QX-314 significantly decreases the upregulated area density of GFAP and p-p38 immunoreactivity. *p<0.05, sham vs. CCI-ION group. #p<0.05, CCI-ION vs. QX-314+CCI-ION groups.
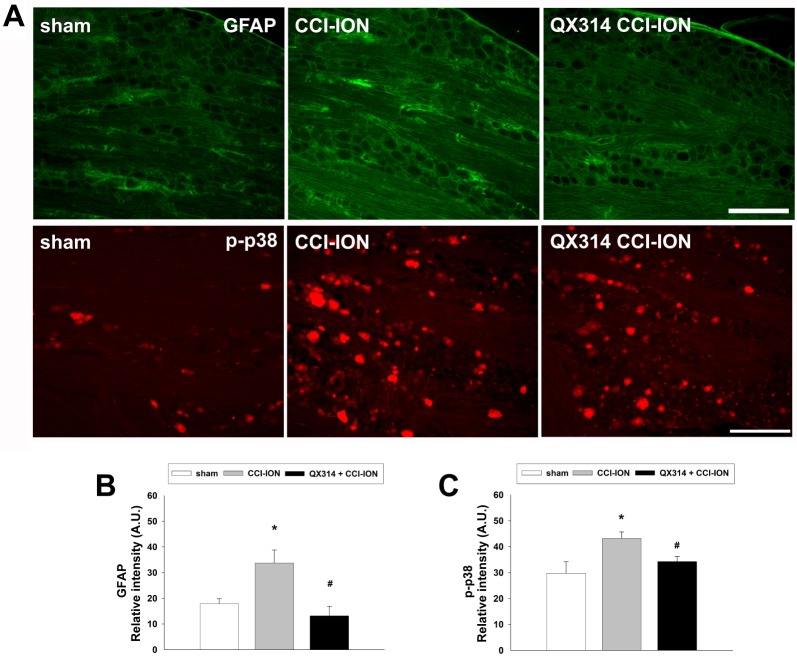
Fig. 7
Double immunostaining for p-p38 with NeuN (a marker of neuron) and GFAP (a marker of satellite cell) to determine the localization of p-p38 in the trigeminal ganglion.
The double immunofluorescence signals revealed a co-localization of p-p38 with NeuN but not with GFAP. Scale bar, 100 µm.
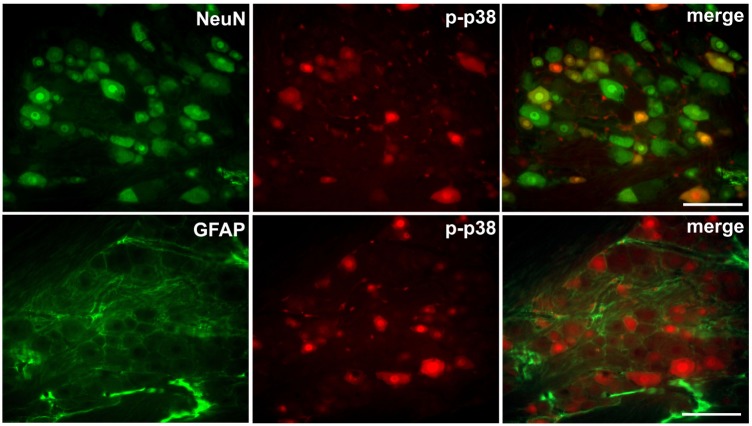
Fig. 8
The effects of preemptive analgesia on the expression of Navs1.3, 1.7, 1.8, and 1.9 in the trigeminal ganglion.
CCI-ION significantly increased the expression of Nav1.3, 1.7, and 1.9 on POD 7 but did not affect the expression of Nav1.8. QX-314-induced preemptive analgesia significantly reduced the Nav1.3, 1.7, and 1.9 upregulation observed in rats following CCI-ION. GAPDH was used as an internal control. *p<0.05, naive vs. CCI-ION group. #p<0.05, CCI-ION vs. QX-314+CCI-ION groups. There were 6 animals in each group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download