Abstract
Intracellular Ca2+ mobilization is closely linked with the initiation of salivary secretion in parotid acinar cells. Reactive oxygen species (ROS) are known to be related to a variety of oxidative stress-induced cellular disorders and believed to be involved in salivary impairments. In this study, we investigated the underlying mechanism of hydrogen peroxide (H2O2) on cytosolic Ca2+ accumulation in mouse parotid acinar cells. Intracellular Ca2+ levels were slowly elevated when 1 mM H2O2 was perfused in the presence of normal extracellular Ca2+. In a Ca2+-free medium, 1 mM H2O2 still enhanced the intracellular Ca2+ level. Ca2+ entry tested using manganese quenching technique was not affected by perfusion of 1 mM H2O2. On the other hand, 10 mM H2O2 induced more rapid Ca2+ accumulation and facilitated Ca2+ entry from extracellular fluid. Ca2+ refill into intracellular Ca2+ store and inositol 1,4,5-trisphosphate (1 µM)-induced Ca2+ release from Ca2+ store was not affected by 1 mM H2O2 in permeabilized cells. Ca2+ efflux through plasma membrane Ca2+-ATPase (PMCA) was markedly blocked by 1 mM H2O2 in thapsigargin-treated intact acinar cells. Antioxidants, either catalase or dithiothreitol, completely protected H2O2-induced Ca2+ accumulation through PMCA inactivation. From the above results, we suggest that excessive production of H2O2 under pathological conditions may lead to cytosolic Ca2+ accumulation and that the primary mechanism of H2O2-induced Ca2+ accumulation is likely to inhibit Ca2+ efflux through PMCA rather than mobilize Ca2+ ions from extracellular medium or intracellular stores in mouse parotid acinar cells.
In the salivary gland, agonist-induced Ca2+ mobilization is the initial cellular event for fluid and amylase secretions [1]. Cytosolic Ca2+ can be mobilized from both external fluid and internal stores, and then rapidly eliminated to internal store and external space in parotid acinar cells [2]. It has been reported that oxidative stress is involved in salivary dysfunction caused by drugs and irradiation [3]. Reduction of submandibular saliva secretion has been observed in the rat treated with lead acetate, which induces oxidative stress [4]. Irradiation-induced hypofunction of the salivary glands has been believed to be involved with oxidative stress [5]. Furthermore, antioxidants have a protective effect on oxidative stress-induced salivary dysfunctions [67]. The impairment of salivary secretion in Sjogren's syndrome, an autoimmune disease which progressively destroys salivary glands, has been known to be generated by oxidative stress and be related to intracellular Ca2+ accumulation in parotid acinar cells [89].
Although reactive oxygen species (ROS) are normally generated from partial reduction of oxygen during the aerobic respiration, they cause oxidative damage to various biological molecules, thereby disrupting normal cellular function and integrity [1011]. The superoxide (O2·−), hydrogen peroxide (H2O2) and hydroxyl radical (OH·) are considered as primary ROS which interact with ion transporters in surface and internal membrane [1213]. ROS are controlled by intracellular antioxidant enzymes and free radical scavengers which protect cells from oxidative stress under physiological conditions [14]. However, the imbalanced states by excessive production of ROS or reduction of antioxidants leading to morphological and functional damage of cells [15]. Although hydrogen peroxide-induced oxidative stress are correlated with overloaded intracellular Ca2+ levels, the mechanism of sustained Ca2+ overload has still been unclear due to cell-to-cell difference in Ca2+ transport molecules as shown by the following evidence: 1) The enhanced Ca2+ release from intracellular store [161718], 2) The stimulated Ca2+ entry from extracellular medium [19202122] and 3) The attenuated Ca2+ efflux by inactivation of plasma membrane Ca2+-ATPase (PMCA) or sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [232425] in various cell types. In the present study, we have therefore characterized the effect of hydrogen peroxide on intracellular Ca2+ signals and the underlying mechanism of Ca2+ accumulation in mouse parotid acinar cells. Here we report that hydrogen peroxide could accumulate intracellular Ca2+ by reducing Ca2+ efflux through PMCA, rather than by enhancing Ca2+ mobilization from extracellular fluid or intracellular store in pathological conditions.
Collagenase P was purchased from Roche Diagnostics GmbH (Mannheim, Germany), fura-2/AM and magfura-2/AM were from Thermo Fisher Scientific (Waltham, MA, USA), inositol 1,4,5-trisphosphate (InsP3) was from Enzo Life Sciences (Farmingdale, NY, USA) and thapsigargin (TG) was from Tocris (Avonmouth, BS, UK). All other materials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Small cluster of parotid acinar cells (8–15 cells per experiment) were freshly isolated by collagenase digestion as described previously [26]. Briefly, the parotid gland was removed from male Balb/c mice (8–10 weeks) after CO2 asphyxiation and cervical dislocation. The tissues were enzymatically digested with collagenase P in HEPES-buffered physiological saline for 30 min following gentle agitation. After isolation, parotid acinar cells were suspended in HEPES-buffered physiological saline containing 137 mM NaCl, 4.7 mM KCl, 0.56 mM MgCl2, 1 mM Na2HPO4, 1.28 mM CaCl2, 10 mM HEPES and 5.5 mM glucose (PH 7.4) until ready for use. To ensure examination in a Ca2+-free condition, HEPES-buffered physiological saline containing no added Ca2+ was replaced with 5 mM ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetra acetic acid (EGTA). All experimental procedures were performed in accordance with the Guideline for the Care and Use of Laboratory Animal provided by NIH. All experiments adhered to Konyang University policies regarding the care and use of animals.
The isolated parotid acinar cells were loaded with 5 µM Fura-2/AM for 1 h at room temperature for measurements of intracellular Ca2+. Fura-2/AM loaded cells were mounted on cover glass in the perfusion chambers. Acinar cells were continuously perfused with HEPES-buffered physiological saline. The perfusion rate (1 ml/min) was controlled using an electronic perfusion system (Warner Instrument, CT, USA). Intracellular Ca2+ imaging was conducted using a TILL Photonics imaging system. Fura-2/AM loaded cells were excited alternately with light at 340 nm and 380 nm using a Polychrome V monochrometer (TILL Photonics, CA, USA). Fluorescence images emitted at 510 nm were captured using a Cool-SNAP HQ2 camera (Photometrics, AZ, USA) attached to inverted microscope (Olympus Corp., Tokyo, JP).
Fura-2 is known to have a high affinity with Mn2+, and thereby fura-2 fluorescence is easily quenched by binding to Mn2+. Therefore, we used this property of Mn2+ to quench fura-2 fluorescence as an indicator of Ca2+ influx through plasma membrane. Fura-2/AM loaded cells were perfused with HEPES-buffered physiological saline. The perfusion solution was then switched to 1 mM Mn2+-containing solution without EGTA or Ca2+ for 10 min to observe the extent of Mn2+ entry. Fura-2/AM loaded cells were excited with light at 360 nm, a wavelength insensitive to intracellular Ca2+ changes. Fluorescence images emitted at 510 nm were captured. Mn2+ quenching the fluorescence signal was normalized using values determined by treatment of 20 µM β-escin to permeabilize cell membrane at the end of the experiments.
The isolated acinar cells were loaded with 3 µM magfura-2/AM for 1 h at room temperature and then attached on cover glass in the perfusion chambers. Cells were permeabilized by perfusion with 20 µM β-escin for 2 min in intracellular medium (ICM) containing 19 mM NaCl, 125 mM KCl, 10 mM HEPES and 1 mM EGTA (pH 7.3) as described previously [27]. To remove intracellular dye, the permeabilized cells were washed with ICM no containing β-escin for 15 min. Intracellular Ca2+ stores were subsequently refilled with Ca2+ by activation of SERCA. To activate SERCA, cells were perfused with ICM containing 0.650 µM CaCl2 (free Ca2+=200 nM), 3 mM Na2ATP and 1.4 mM MgCl2. After the stores were refilled with Ca2+, SERCA activity was effectively inactivated by removal of Mg2+ from ICM. The free Ca2+ was constantly maintained at 200 nM throughout all experiments. Fluorescence images emitted 505 nm were captured following alternate excitation at 340 nm and 380 nm using a TILL Photonics imaging system.
All results were presented as mean±S.E. Data were analyzed using the Student's t test. Differences were considered significant when the p value was less than 0.05. Ca2+ refill rates and Ca2+ release rates were estimated by fitting the changing fluorescence to a single exponential function. Relative Ca2+ entry and efflux were normalized to maximum value in each experiments using Origin program.
Initial experiments were performed to investigate the effect of H2O2 on the intracellular Ca2+ level in parotid acinar cells. The change of intracellular Ca2+ concentration was monitored in various concentrations of H2O2 (0.1–10 mM) in the presence of 1.28 mM extracellular Ca2+ in intact cells. As shown in Fig. 1A, the perfusion of H2O2 for 10 min resulted in slow increases of intracellular Ca2+ concentrations. The significant Ca2+ elevations were observed from 1 mM of H2O2 and more rapid Ca2+ accumulation was observed in 10 mM H2O2. After the cessation of H2O2 perfusion, the augmented Ca2+ was nearly returned to baseline at 1 mM of H2O2. Contrastively, the sustained Ca2+ increase was observed at 3 mM and 10 mM of H2O2, even if H2O2 was removed from the perfusate (Fig. 1B). These results suggest that H2O2 could remarkably accumulate in intracellular Ca2+ and that an excess dose of H2O2 could irreversibly alter Ca2+ homeostasis in parotid acinar cells.
Next, we compared the intracellular Ca2+ accumulation in the presence and the absence of extracellular Ca2+ to confirm whether H2O2 could facilitate Ca2+ entry from the extracellular medium. Intracellular Ca2+ accumulation steadily increased during treatment of H2O2 in both normal Ca2+ and Ca2+-free mediums (Fig. 2A). Although, a slight difference was detected in initial values of Ca2+ accumulation, the final values of Ca2+ accumulation showed no difference as shown in Fig. 2B (values at 300 s; 0.064±0.012 Δ ratio vs 0.034±0.007 Δ ratio, values at 600 s; 0.123±0.023 Δ ratio vs 0.122±0.015 Δ ratio, presence and absence of extracellular Ca2+, respectively). Thus, the entire Ca2+ accumulation was observed regardless of external Ca2+ existence.
In another experiment, the Mn2+ quenching test was performed to confirm Ca2+ entry. As shown in Fig. 3A, the perfusion of 1 mM and 3 mM H2O2 for 10 min failed to facilitate quenching of fura-2 fluorescence, whereas 10 mM 2O2 markedly accelerated quenching of fura-2 fluorescence. The relative Ca2+ entries at the end of the experiments were 57.14±8.73%, 62.99±6.03%, 63.56±6.79% and 86.21±1.77% in control, 1 mM, 3 mM and 10 mM H2O2-treated groups, respectively (Fig. 3B). These data indicate that the primary origin of accumulated Ca2+ induced by 1 mM H2O2 may not come from extracellular fluid because Ca2+ accumulation was still observed in Ca2+-free solution and Mn2+ quenching of fura-2 fluorescence was not facilitated in the presence of 1 mM H2O2. Therefore, we used a concentration of 1 mM H2O2 in the following experiments to identify the primary mechanism of Ca2+ accumulation. The next experiment was planned to evaluate whether H2O2 can influence Ca2+ transport through intracellular Ca2+ stores membrane.
To determine the influence of H2O2 on Ca2+ transport through endoplasmic reticulum (ER) membrane, we employed unidirectional fluorescent ER Ca2+ measurements in permeabilized cells. Parotid acinar cells were loaded with a low-affinity Ca2+ dye magfura-2/AM and then permeabilized with β-escin to release the cytosolic dye. As shown in Fig. 4A, the perfusion with intracellular medium containing CaCl2, MgCl2 and ATP resulted in an increase of the fluorescence ratio that indicates an effective Ca2+ refill into the ER stores. In these conditions, the perfusion of 1 mM H2O2 failed to change Ca2+ refill rate into the ER stores (Fig. 4B). After the stores were refilled with Ca2+, MgCl2 was eliminated from the buffer to provide SERCA inactivation. Application of 1 µM InsP3 caused significant Ca2+ release from the stores, but H2O2 had no effect on InsP3-induced Ca2+ release rates (Fig. 4C). These findings indicate that 1 mM H2O2 led to neither Ca2+ refill nor InsP3-induced Ca2+ release through the ER membrane.
To confirm the effect of H2O2 on PMCA activity, we evaluated the effect of H2O2 in Ca2+-free conditions during the depletion of Ca2+ from the ER store by the treatment of 1 µM TG, a SERCA blocker. As shown in Fig. 5A, TG induced a transient Ca2+ elevation that indicates spontaneous Ca2+ release from the ER stores, and these responses were completely returned to baseline, which means there was Ca2+ efflux by plasma membrane Ca2+-ATPase (PMCA) in control experiments. When H2O2 was treated with TG, the peak time of Ca2+ elevation was delayed (Fig. 5B, control; 24.83±2.76 s vs. H2O2; 37.17±3.17 s) and Ca2+ efflux was not fully returned to the control value in 1 mM H2O2-treated group (Fig. 5C, control; 103.95±7.62% vs. H2O2; 39.58±9.33%).
To further test the effect of H2O2 on PMCA activity in another set of experiments, Ca2+ store were initially depleted with 1 µM TG, and then Ca2+ entry and Ca2+ efflux was fully stimulated by adding and removing extracellular 1.28 mM Ca2+ in intact cells, respectively. In the control experiment, as shown in Fig. 6A, the addition of extracellular Ca2+ remarkably stimulated Ca2+ entry, and the elimination of extracellular Ca2+ resulted in a clear extrusion of intracellular Ca2+ to external space. In 1 mM H2O2-treated cells, the elevated intracellular Ca2+ level was not returned to baseline by withdrawal of extracellular Ca2+. That means the extrusion of intracellular Ca2+ was markedly disrupted by treatment of 1 mM H2O2. Additionally, the perfusion of antioxidants with H2O2, either 30 µg/ml catalase or 2 mM dithiothreitol, completely protected the diminished Ca2+ efflux in TG-treated intact acinar cells (Fig. 6B). These evidences suggest that H2O2 may induce Ca2+ accumulation by inhibition of PMCA activity in mouse parotid acinar cells.
The present study provides evidence that the reactive oxygen species H2O2 accumulates cytosolic Ca2+ by attenuating Ca2+ efflux through PMCA rather than by mobilizing the Ca2+ from the extracellular space or intracellular Ca2+ store in mouse parotid acinar cells. Cytosolic free Ca2+ plays a crucial role in the salivary secretion of parotid acinar cells, and Ca2+ can be mobilized from both the external fluid and the internal Ca2+ stores such as endoplasmic reticulum, acidic store and mitochondria to elicit physiological responses [282930]. Acetylcholine, a major agonist in the parotid gland, is known to initially mobilize Ca2+ from internal stores through activation of InsP3 receptors, and to subsequently activate the store-operated Ca2+ entry from the external medium to refill the depleted stores [3132]. After Ca2+ mobilization, cytosolic Ca2+ was rapidly eliminated to internal store and external space by SERCA and PMCA, respectively. Since the accumulation of intracellular Ca2+ causes cellular toxicity, including apoptosis and necrosis, basal intracellular Ca2+ concentrations are finely regulated to under about 10,000 folds compare to the extracellular space [33]. The balance between Ca2+ mobilization and Ca2+ elimination are regulated by various Ca2+ transporters expressed both in plasma membrane and ER membrane [34]. Oxidative stress is well known risk factor which induces cellular dysfunction of several tissues and organs [1011], and oxidants-induced cellular dysfunction are closely linked with intracellular Ca2+ accumulation [1213]. In this study, when parotid acinar cells were exposed to 1 mM H2O2 in normal Ca2+ buffer, there was a significant intracellular Ca2+ accumulation, and the elevated Ca2+ was nearly returned to baseline after the cessation of H2O2 perfusion. Generally, H2O2 is known to accumulate cytosolic Ca2+ without acute cell death at concentrations from 10 µM to 5 mM in various cell types [16171819202122232425]. Thus, mouse parotid acinar cells are thought to be relative resistance to H2O2.
To confirm the underlying mechanism of H2O2-induced Ca2+accumulation, we measured Ca2+ transport through plasma membrane and ER membrane. Actually, H2O2-induced Ca2+ entry through plasma membrane is important for intracellular Ca2+ accumulation, and transient-receptor potential channels and store-operated Ca2+ channels are known to involved in Ca2+ overload in various cell types [19202122]. However, in our study, the entire Ca2+ accumulation induced by 1 mM H2O2 was not suppressed by the elimination of extracellular Ca2+ in intact acinar cells and Mn2+ quenching property was not facilitated by the perfusion of 1 mM H2O2. Since 10 mM H2O2 produced more rapid cytosolic Ca2+ elevation and accelerated Mn2+ quenching fura-2 fluorescence, higher concentrations of H2O2 may be required to promote Ca2+ influx in parotid acinar cells. Moreover, H2O2 failed to stimulate InsP3-induced Ca2+ release for measurement Ca2+ transport through ER membrane in permeabilized acinar cells. These results suggest that H2O2 could accumulate cytosolic Ca2+ irrelevant of Ca2+ entry from external fluid and Ca2+ release from ER stores. Since H2O2 could mobilize Ca2+ from other TG-insensitive intracellular stores such as mitochondria [3536], we evaluated the effect of H2O2 in Ca2+-free conditions after the depletion of Ca2+ from the ER store by the pretreatment of 1 µM TG (Supplementary Fig. 1). TG induced a transient Ca2+ elevation, and these responses were completely returned to baseline. After return to baseline, the perfusion of 1 mM H2O2 failed to increase the Ca2+ release. It has been proposed that mitochondrial Ca2+ release by H2O2 could conceivably contribute to Ca2+ accumulation when cytosolic Ca2+ is high, since H2O2 evoked cytosolic Ca2+ elevation when cells were pre-stimulated by cholecystokinin (CCK) but failed to evoke cytosolic Ca2+ elevation without prestimulation of CCK in ER Ca2+ stores were depleted pancreatic acinar cells [24]. Thus, 1 mM H2O2-induced Ca2+ accumulation is not due to mobilize Ca2+ from other intracellular Ca2+ stores or mitochondria in our study. On the other hand, when TG were treated with 1 mM H2O2 in the absence of extracellular Ca2+, cytosolic Ca2+ elevation was delayed and enhanced Ca2+ was not fully recovered to baseline. Actually, slow intracellular Ca2+ elevation by SERCA inhibition was likely due to spontaneous release or leak of Ca2+ from the ER stores, and slow Ca2+ decline was due to extrusion of Ca2+ to the external space by PMCA or Na+/Ca2+ exchanger (NCX) activation [37]. Although, NCX are also known to sensitive to oxidants [1213], there is no evidence of the expression and the participation of NCX for Ca2+ extrusion in parotid acinar cells at the present time. Furthermore, Ca2+ extrusion was not affected when Na+ was replaced with NMDG+ to NCX inactivation in our preliminary study (data not shown). Since the removal of elevated Ca2+ from cytosol to the extracellular site was carried out mainly by PMCA under inhibition of SERCA by TG treatment in parotid acinar cells, this finding strongly suggests that H2O2 effectively suppressed PMCA activity. In another set of experiment, we further investigated the effect of H2O2 on Ca2+ efflux through PMCA. PMCA activity was assessed by a Ca2+ decrease to baseline by removing extracellular Ca2+ after store-operated Ca2+ entry was fully stimulated by adding extracellular Ca2+ in TG-treated cells. Here we show that the extrusion of intracellular Ca2+ was markedly disrupted by pretreatment of 1 mM H2O2 in TG-treated intact acinar cells. Actually, the initial Ca2+ levels were slightly higher in H2O2-treated cells than in control cells. These results were considered caused by blocking of Ca2+ efflux through PMCA, rather than by enhancing of the Ca2+ entry through activation of store-operated Ca2+ channels. Furthermore, the inhibitory effects of H2O2 on Ca2+ efflux were strongly protected by the adding of catalase, an enzyme degrading hydrogen peroxide, and DTT, a sulfhydryl reducing agent.
In fact, it has been reported that functionally important sulfhydryl group are present within PMCA and SERCA molecules that is localized within the catalytic and controlling centers of Ca2+-ATPase [383940]. We previously reported that H2O2 accumulates intracellular Ca2+ by attenuating SERCA activity in pancreatic acinar cells [25]. Interestingly, in this study, H2O2 failed to change Ca2+ refilling into the ER store through SERCA by the application of Ca2+ and Mg-ATP in permeabilized parotid acinar cells. As shown in supplement Fig. 2, three folds higher concentration of H2O2 are need to inhibit Ca2+efflux through PMCA in pancreatic acinar cells compared to parotid acinar cells. Ca2+-ATPase has distinct isoforms with different expression and regulation properties that caused diversity of Ca2+ signaling in various cell types [41]. It has been known that distinct Ca2+-ATPase isoforms have different sensitivity to ROS due to locational difference of sulfhydryl group [42,43,44]. Thus, the different sensitivities of Ca2+-ATPase to H2O2 between parotid and pancreatic acinar cells are thought to be due to differences in expression and regulation of Ca2+-ATPase. In immunofluorescence study, PMCA1 is distributed throughout the plasma membrane, PMCA2 is localized to the basolateral membrane and PMCA4 is localized to the apical membrane in parotid acinar cell [45]. Even though PMCAs are dominantly expressed in apical membrane in pancreatic acinar cells [4647], isoform specific distributions are not clear at the present time. Although the further studies are need to elucidate this issues, we predict from the present results that H2O2 could lead to accumulate cytosolic Ca2+ by attenuation of Ca2+ efflux through the oxidation of functional sulfhydryl groups of PMCA in parotid acinar cells.
From the above results, we concluded that H2O2 can accumulate intracellular Ca2+ by attenuating Ca2+ efflux through PMCA, rather than by mobilizing Ca2+ from extracellular medium or intracellular stores in parotid acinar cells. Thus the primary target for H2O2 excessively generated in pathological conditions is considered PMCA in mouse parotid acinar cells.
Figures and Tables
Fig. 1
Hydrogen peroxide (H2O2) produced intracellular Ca2+ accumulation in intact parotid acinar cells.
(A) Effects of various concentrations (0.1–10 mM) of H2O2 (filled circles) for 10 min on Ca2+ accumulation in the presence of normal extracellular Ca2+. (B) The peak (open bars) and recovery (filled bars) values of intracellular Ca2+ accumulation after 10 min of the perfusion and the removal of H2O2. The changes of the 340/380 ratio were expressed as means±SE obtained from at least seven separate experiments. The perfusion of H2O2 resulted in slow increases of intracellular Ca2+ concentrations. H2O2 at concentration of 1 mM effectively accumulate intracellular Ca2+ and nearly recovered to baseline after withdraw of H2O2. The sustained Ca2+ increase was still observed at higher concentration over 3 mM of H2O2 even if H2O2 was removed from the perfusate. The relatively rapid Ca2+ accumulation was observed in 10 mM H2O2. Asterisk indicates the recovery value obtained at 10 min after termination of H2O2 treatment is significantly different from the peak value obtained during H2O2 treatment (p<0.05).

Fig. 2
H2O2 still enhanced Ca2+ accumulation in the absence of extracellular Ca2+ in intact parotid acinar cells.
(A) Effects of 1 mM H2O2 on Ca2+ accumulation in the absence (open circles) or the presence (filled circles) of normal extracellular Ca2+. (B) Intracellular Ca2+ accumulation at 300 s and 600 s after perfusion of 1 mM H2O2 in the absence (open bars) or the presence (filled bars) of normal extracellular Ca2+. The values were expressed as means±SE obtained from seven (control) and five (H2O2) experiments. Although a slight difference was detected in initial values of Ca2+ accumulation at 300 s, the entire Ca2+ accumulation at 600 s was no difference. Asterisk indicates the value obtained in Ca2+-free buffer is significantly different from the corresponding value obtained in normal Ca2+ buffer (p<0.05).

Fig. 3
Effects of H2O2 on Ca2+ entry using Mn2+ quenching test in intact parotid acinar cells.
(A) Effects of various concentrations (1–10 mM) of H2O2 for 10 min on normalized Ca2+ entry. (B) Effects of H2O2 on relative Ca2+ entry at the end of experiments. The values were expressed as means±SE obtained from at least six separate experiments. H2O2 at the concentration of 1 mM (open squares) and 3 mM (open triangles) failed to facilitate Ca2+ entry, whereas 10 mM H2O2 (open diamonds) remarkably accelerated Ca2+ entry through plasma membrane. Asterisk indicates the value obtained in H2O2-treated experiments (filled bars) is significantly different from the corresponding value obtained in control experiments (open bar) (p<0.05).

Fig. 4
H2O2 failed to modulate Ca2+ transport through endoplasmic reticulum (ER) membrane in permeabilized parotid acinar cells.
(A)The effect of H2O2 on Ca2+ refill and InsP3-induced Ca2+ release through ER membrane in permeabilized cells. (B) The effect of H2O2 on Ca2+ refill rates into intracellular Ca2+ stores. (C) The effect of H2O2 on InsP3-induced Ca2+ release from Ca2+ stores. The values were expressed as means±SE obtained from five (control) and six (H2O2) experiments. ER Ca2+ stores were loaded with the buffer containing MgCl2, Na2ATP and CaCl2. After Ca2+ loading, MgCl2 was eliminated to SERCA inactivation at 60 s prior to 1 µM InsP3 application (open circles and bars). The same procedure was repeated in H2O2-treated permeabilized cells (filled circles and bars). The perfusion of H2O2 resulted in change neither Ca2+ refill rate nor InsP3-induced Ca2+ release rate through ER membrane in permeabilized cells.

Fig. 5
H2O2 attenuated Ca2+ efflux in thapsigargin (TG)-treated intact acinar cells.
(A) The effect of H2O2 on Ca2+ efflux during the depletion of ER Ca2+ store by TG treatment in Ca2+-free buffer. (B) The effect of H2O2 on the peak time of intracellular Ca2+ elevation. (C) The effect of H2O2 on the relative Ca2+ efflux at the end of experiments. The values were expressed as means±SE obtained from seven (control) and five (H2O2) experiments. When H2O2 was treated with TG (filled circles and bars), the Ca2+ elevation was delayed and Ca2+ efflux was not fully recovered to baseline compared with control values (open circles and bars). Asterisks indicate the value obtained in H2O2-treated experiments is significantly different from the corresponding value obtained in control experiments (p<0.05).
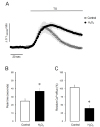
Fig. 6
H2O2 suppressed Ca2+ efflux through PMCA in intact parotid acinar cells.
(A) The effect of H2O2 and antioxidants on Ca2+ efflux through PMCA. (B) The effect of H2O2 and antioxidants on relative Ca2+ efflux at the end of experiments. The values were expressed as means±SE obtained from at least five separate experiments. Ca2+ store were initially depleted with 1 µM TG, and then Ca2+ entry and Ca2+ efflux was fully stimulated by adding and removing extracellular 1.28 mM Ca2+. Application of H2O2 (filled circles and bar) resulted in remarkable inhibition of Ca2+ efflux through PMCA compared with control values (open circles and bar) in TG-treated acinar cells. The antioxidants, 30 µg/ml catalase (filled triangles and gray bar) or 2 mM dithiothreitol (DTT, filled diamonds and gray bar), completely protected these diminished Ca2+ efflux by H2O2 in TG-treated intact acinar cells. Asterisk indicates the value obtained in H2O2-treated experiments is significantly different from the corresponding value obtained in control experiments (p<0.05).

Notes
References
1. Ambudkar IS. Calcium signalling in salivary gland physiology and dysfunction. J Physiol. 2016; 594:2813–2824.

2. Petersen OH. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992; 448:1–51.

4. Abdollahi M, Fooladian F, Emami B, Zafari K, Bahreini-Moghadam A. Protection by sildenafil and theophylline of lead acetate-induced oxidative stress in rat submandibular gland and saliva. Hum Exp Toxicol. 2003; 22:587–592.
5. Acauan MD, Figueiredo MA, Cherubini K, Gomes AP, Salum FG. Radiotherapy-induced salivary dysfunction: Structural changes, pathogenetic mechanisms and therapies. Arch Oral Biol. 2015; 60:1802–1810.

6. Yamada T, Ryo K, Tai Y, Tamaki Y, Inoue H, Mishima K, Tsubota K, Saito I. Evaluation of therapeutic effects of astaxanthin on impairments in salivary secretion. J Clin Biochem Nutr. 2010; 47:130–137.

7. Abedi SM, Yarmand F, Motallebnejad M, Seyedmajidi M, Moslemi D, Ashrafpour M, Bijani A, Moghadamnia A, Mardanshahi A, Hosseinimehr SJ. Vitamin E protects salivary glands dysfunction induced by ionizing radiation in rats. Arch Oral Biol. 2015; 60:1403–1409.

8. Ryo K, Yamada H, Nakagawa Y, Tai Y, Obara K, Inoue H, Mishima K, Saito I. Possible involvement of oxidative stress in salivary gland of patients with Sjogren's syndrome. Pathobiology. 2006; 73:252–260.
9. Pagano G, Castello G, Pallardó FV. Sjøgren's syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials. Free Radic Res. 2013; 47:71–73.

10. Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, Lichtenstein A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997; 22:73–83.

11. Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998; 75:199–212.

12. Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998; 275:C1–24.
14. Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994; 74:139–162.

15. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.

16. Roveri A, Coassin M, Maiorino M, Zamburlini A, van Amsterdam FT, Ratti E, Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch Biochem Biophys. 1992; 297:265–270.

17. Gen W, Tani M, Takeshita J, Ebihara Y, Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001; 96:623–629.
18. Sun L, Yau HY, Lau OC, Huang Y, Yao X. Effect of hydrogen peroxide and superoxide anions on cytosolic Ca2+: comparison of endothelial cells from large-sized and small-sized arteries. PLoS One. 2011; 6:e25432.
19. Giambelluca MS, Gende OA. Hydrogen peroxide activates calcium influx in human neutrophils. Mol Cell Biochem. 2008; 309:151–156.

20. Liu X, Cotrim A, Teos L, Zheng C, Swaim W, Mitchell J, Mori Y, Ambudkar I. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun. 2013; 4:1515.

21. Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014; 21:971–986.

22. Santiago E, Climent B, Muñoz M, García-Sacristán A, Rivera L, Prieto D. Hydrogen peroxide activates store-operated Ca2+ entry in coronary arteries. Br J Pharmacol. 2015; 172:5318–5332.
23. Zaidi A, Barŕon L, Sharov VS, Schöneich C, Michaelis EK, Michaelis ML. Oxidative inactivation of purified plasma membrane Ca2+-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003; 42:12001–12010.
24. Bruce JI, Elliott AC. Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase. Am J Physiol Cell Physiol. 2007; 293:C938–C950.
25. Yoon MN, Kim DK, Kim SH, Park HS. Hydrogen peroxide attenuates refilling of intracellular calcium store in mouse pancreatic acinar cells. Korean J Physiol Pharmacol. 2017; 21:233–239.

26. Park HS, Betzenhauser MJ, Zhang Y, Yule DI. Regulation of Ca2+ release through inositol 1,4,5-trisphosphate receptors by adenine nucleotides in parotid acinar cells. Am J Physiol Gastrointest Liver Physiol. 2012; 302:G97–G104.
27. Choi KJ, Kim KS, Kim SH, Kim DK, Park HS. Caffeine and 2-aminoethoxydiphenyl borate (2-APB) have different ability to inhibit intracellular calcium mobilization in pancreatic acinar cell. Korean J Physiol Pharmacol. 2010; 14:105–111.

28. Gallacher DV, Petersen OH. Stimulus-secretion coupling in mammalian salivary glands. Int Rev Physiol. 1983; 28:1–52.
29. Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003; 31:954–957.
30. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005; 67:445–469.

31. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529.

32. Won JH, Cottrell WJ, Foster TH, Yule DI. Ca2+ release dynamics in parotid and pancreatic exocrine acinar cells evoked by spatially limited flash photolysis. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G1166–G1177.
35. Pariente JA, Camello C, Camello PJ, Salido GM. Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol. 2001; 179:27–35.

36. Baggaley EM, Elliott AC, Bruce JI. Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: role of the mitochondria. Am J Physiol Cell Physiol. 2008; 295:C1247–C1260.
37. Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann N Y Acad Sci. 2007; 1099:226–236.
38. Bellomo G, Mirabelli F, Richelmi P, Orrenius S. Critical role of sulfhydryl group(s) in ATP-dependent Ca2+ sequestration by the plasma membrane fraction from rat liver. FEBS Lett. 1983; 163:136–139.
39. Zaidi A, Barŕon L, Sharov VS, Schöneich C, Michaelis EK, Michaelis ML. Oxidative inactivation of purified plasma membrane Ca2+-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003; 42:12001–12010.
40. Pengpanichpakdee N, Thadtapong T, Auparakkitanon S, Wilairat P. Plasma membrane Ca2+-ATPase sulfhydryl modifications: implication for oxidized red cell. Southeast Asian J Trop Med Public Health. 2012; 43:1252–1257.
41. Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001; 81:21–50.

42. Grover AK, Samson SE, Misquitta CM. Sarco(endo)plasmic reticulum Ca2+ pump isoform SERCA3 is more resistant than SERCA2b to peroxide. Am J Physiol. 1997; 273:C420–C425.
43. Barnes KA, Samson SE, Grover AK. Sarco/endoplasmic reticulum Ca2+-pump isoform SERCA3a is more resistant to superoxide damage than SERCA2b. Mol Cell Biochem. 2000; 203:17–21.
44. Yin D, Kuczera K, Squier TC. The sensitivity of carboxyl-terminal methionines in calmodulin isoforms to oxidation by H2O2 modulates the ability to activate the plasma membrane Ca-ATPase. Chem Res Toxicol. 2000; 13:103–110.
45. Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI. Differential regulation of the apical plasma membrane Ca2+-ATPase by protein kinase A in parotid acinar cells. J Biol Chem. 2007; 282:37678–37693.
46. Belan PV, Gerasimenko OV, Tepikin AV, Petersen OH. Localization of Ca2+ extrusion sites in pancreatic acinar cells. J Biol Chem. 1996; 271:7615–7619.
47. Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997; 272:15771–15776.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download