Abstract
Inhibition of K+ outward currents by linopirdine in the outer hair cells (OHCs) of circling mice (homozygous (cir/cir) mice), an animal model for human deafness (DFNB6 type), was investigated using a whole cell patch clamp technique. Littermate heterozygous (+/cir) and ICR mice of the same age (postnatal day (P) 0 –P6) were used as controls. Voltage steps from –100 mV to 40 mV elicited small inward currents (–100 mV~–70 mV) and slow rising K+ outward currents (–60 mV ~40 mV) which activated near –50 mV in all OHCs tested. Linopirdine, a known blocker of K+ currents activated at negative potentials (IK,n), did cause inhibition at varying degree (severe, moderate, mild) in K+ outward currents of heterozygous (+/cir) or homozygous (cir/cir) mice OHCs in the concentration range between 1 and 100 µM, while it was apparent only in one ICR mice OHC out of nine OHCs at 100 µM. Although the half inhibition concentrations in heterozygous (+/cir) or homozygous (cir/cir) mice OHCs were close to those reported in IK,n, biophysical and pharmacological properties of K+ outward currents, such as the activation close to –50 mV, small inward currents evoked by hyperpolarizing steps and TEA sensitivity, were not in line with IK,n reported in other tissues. Our results show that the delayed rectifier type K+ outward currents, which are not similar to IK,n with respect to biophysical and pharmacological properties, are inhibited by linopirdine in the developing (P0~P6) homozygous (cir/cir) or heterozygous (+/cir) mice OHCs.
Expression of K+ channels is developmentally regulated in cochlear outer hair cells (OHCs). Before hearing onset, delayed rectifier K+ current (IK,emb, IK,neo) [1], inward rectifier K+ current (IK1) [2], small conductance Ca2+ activated K+ current (ISK) [34], and K+ current activated at negative potentials (IK,n) are sequentially expressed in murine cochlear OHCs [5]. The reports, which suggest that the timely expression of K+ channels is important for the functional maturation of cochlear hair cells [156], raise a possibility that K+ channel expression in the early stage might be different in the cochlear hair cells of genetically abnormal animals, such as mouse models for human deafness.
We reported [7] the characteristics of K+ outward currents recorded in the OHCs of immature circling mouse (P0~P6), a mouse model for human deafness (DFNB6 type) [89], as a scanning electron microscope study demonstrated that the stereocilliary defects of OHCs were observable as early as P10 and were more severe in the OHCs than in the inner hair cells (IHCs) at P18 in circling mice [10].
This is a study following the previous study which demonstrated the subtle biophysical and pharmacological differences between circling mice (homozygous (cir/cir) mice) and their littermates (heterozygous (+/cir) mice). While investigating the pharmacological aspects of K+ outward currents in the previous study [7], we tested TEA and 4-AP only, not linopirdine, a known inhibitor of IK,n, as IK,n is not reported to develop before P6 in mice OHCs [5]. However, from succeeding experiments using linopirdine, we found significant inhibitory effects of linopirdine on K+ outward currents of P0~P6 heterozygous (+/cir) or homozygous (cir/cir) mice and we will discuss this finding in this report.
Female heterozygous (+/cir) mice were mated with male homozygous (cir/cir) mice (circling mice), and their offspring were used in this study. The circling mice strain has been maintained for more than 10 generations by breeding between female heterozygous (+/cir) mice and their male siblings (homozygous (cir/cir) mice) at the Animal Facility of Dankook University since 2007. The data presented were obtained from pups between P0 and P6. Genotypes were assessed by polymerase chain reaction analysis according to our previous report [11]. The Dankook University Institutional Animal Care and Use Committee (DUIAC) approved this study.
After the mice received deep anesthesia with isoflurane, their cochleae were removed and dissected in an ice-cold solution composed of (in mM): NaCl (124), KCl (5), KH2PO4 (1.25), glucose (10), NaHCO3 (26), CaCl2 (2), MgSO4 (1.3), and sucrose (20). The pH was 7.4 when aerated with 95% O2 and 5% CO2 and the osmolarity was about 305 mOsm. After removing the bony part and modiolus, the dissected cochleae were transferred to a submersion-type chamber mounted on an upright microscope and immobilized under a nylon mesh fixed to a stainless steel ring. The chamber was perfused continuously with the same solution used during preparation.
Whole cell currents were recorded from OHCs located in the middle turn of the cochlea. All experiments were performed at room temperature using an EPC-8 (HEKA, Lambrecht, Germany) amplifier. Electrodes (3~5 MΩ) were filled with a solution containing (in mM): K-gluconate (108), EGTA (0.6), KCl (5), HEPES (10), Na2GTP (0.3), MgATP (1), KOH (30), sucrose (47), and QX 314 (5). All chemicals except for QX 314 (Tocris) were purchased from Sigma Chemicals Co., unless otherwise stated. Except for fast capacitance cancellation at the cell-attached stage, series resistance was not compensated and no corrections were made for the liquid junction potentials. The data were filtered at 5 kHz (EPC-8, HEKA), digitized at 10 kHz, and stored in the computer via a home-made program (R-Clamp 1.23) for offline analysis. The stored data were analyzed using Clampfit 9.0 (Molecular Devices), Origin 7.0 (Origin Lab). Data were expressed as the mean±SEM. An independent t-test was used for comparisons. Null hypotheses of no difference were rejected if p values were <.05.
Depolarizing voltage steps from –100 mV to 40 mV (holding potential: –60 mV) evoked small inward (<100 pA) and slowly inactivating outward K+ currents in the OHCs of all mice tested. In Fig. 1A, only the data obtained from ICR mice was presented, as those for heterozygous (+/cir) and homozygous (cir/cir) were already presented in the previous paper [7]. All the K+ outward currents were similar to that reported previously (a delayed rectifier-type K+ current, IK,neo [1], for neonatal cells). The outward currents activated at potentials close to –50 mV in all mice tested. Small inward currents did not show any active current decay, which was usually observed in mice OHCs older than P9 [5] even at –120 mV (Data not shown). The current density-voltage relation of ICR mice is presented in Fig. 1B. The current density measured at 40 mV was 212.6±20.8 pA/pF in ICR mice (n=11). (In the previous paper [7], they were 227.1±18.9 pA/pF (n=16) in heterozygous (+/cir) mice and 238.9±14.3 pA/pF (n=22) in homozygous (cir/cir) mice).
Activation curves were derived from tail currents at –30 mV. The activation curves shown in Fig. 1C were obtained by plotting the normalized tail currents against different prepulse potentials from –90 mV to 40 mV. Data were fitted by a modified first-order Boltzmann equation: y=A2+(A1–A2)/{1+exp(V-Vhalf/S)} where y is the normalized peak current, A2 is the minimal normalized peak current, A1 is the maximal normalized peak current, Vhalf is the potential of half-maximal activation, V is the commanding potential, and S is the slope factor representing the voltage sensitivity of activation. Vhalf was –20.7±4.7 mV (n=7, ICR mice), –21.3±1.7 mV (n=9, heterozygous (+/cir) mice), and –20.8±3.3 mV (n=11, homozygous (cir/cir) mice). The slope factor was 9.8±0.9 mV (n=7, ICR mice), 13.8±1.8 (n=9, heterozygous (+/cir) mice) and 11.4±1.0 mV (n=11, homozygous (cir/cir) mice). They were not significantly different. In the previous paper, the reported values of Vhalfs were –8.1 (heterozygous (+/cir) mice) and –17.2 (homozygous (cir/cir) mice) [7]. In the previous paper, the activation curves were derived by calculating conductance (peak current/(Vcommand–Vholding)) at each commanding potential (Vholding=–60 mV), while in this paper, we used the tail current analysis. This might be the cause of the difference in Vhalfs.
The above results indicated that biophysical properties of OHC K+ outward currents of heterozygous (+/cir), homozygous (cir/cir), and ICR mice were similar but quite different from those of IK,n reported in OHCs of older mice aged more than P9 [5] in which 1) hyperpolarizing voltage steps elicited only small inward K+ currents, 2) they did not show any active decay and 3) outward currents activated at potentials close to –50 mV, while they activated at potentials negative to –100 mV in OHCs older than P9 when IK,n developed fully, which suggested that K+ outward currents do not have the IK,n component. In spite of this, we tested the effects of linopirdine (1, 10, and 100 µM), a known blocker of IK,n to confirm that IK,n did not develop early in OHCs of homozygous (cir/cir) or heterozygous (+/cir) mice.
K+ currents were elicited by a series of voltage steps from –60 mV to 40 mV (holding potential: –60 mV) and the current amplitudes were measured at the end of the voltage steps (200 ms) for analysis. The responses of K+ outward currents were not simple but were categorized into 2 or 3 groups (severe, moderate, mild) according to the extent of inhibition by linopirdine in homozygous (cir/cir) or heterozygous (+/cir) mice. Among 13 cells of homozygous (cir/cir) mice, 5 cells showed a severe block, 4 cells showed a moderate block, and 4 cells showed a mild block. In 5 cells showing a severe block, the K+ current recorded at 40 mV was reduced to 51.0±1.6% (n=5), 33.1±6.1% (n=5), and 15.7±2.1% (n=5) of the control by 1 µM, 10 µM, and 100 µM linopirdine, respectively (Fig. 2A). In 4 cells showing a moderate block, the corresponding values were 69.5±5.3% (n=4), 56.7±3.0% (n=4), and 33.8±2.3% (n=4) of the control by 1 µM, 10 µM, and 100 µM linopirdine, respectively (Fig. 2B). Among 4 cells showing a mild block, we succeeded in observing a 100 µM response only in one cell. In the remaining 3 cells, we only observed the effects of linopirdine up to 10 µM. The K+ current at 40 mV was reduced to 91.7±4.7% (n=3, 1 µM) and 85.7±1.0% (n=3, 10 µM). The 100 µM response in one cell is shown in Fig. 2C. The currents at 40 mV were reduced to 90.5% (1 µM), 85.7% (10 µM), and 42.8% (100 µM). Linopirdine was not effective at hyperpolarizing voltages (–100 mV~–70 mV). Fig. 2D shows the representative data. No inhibition at the hyperpolarizing voltages was also observed in heterozygous (+/cir) and ICR mice (data not shown).
Among 10 cells of heterozygous (+/cir) mice, 4 cells showed a severe block and 6 cells showed a moderate block. In the OHCs of heterozygous (+/cir) mice showing a severe block, the current was reduced to 62.1±3.1% (n=4, 1 µM), 25.0±4.5% (n=4, 10 µM), and 8.9±3.5% (n=4, 100 µM) (Fig. 3A), while in the OHCs showing a moderate block, the values were 75.7±6.6% (n=6, 1 µM) and 55.4±3.4% (n=6, 10 µM) (Fig. 3B). We failed to record a 100 µM response in cells showing a moderate block.
In ICR mice, linopirdine responses were quite different from those in homozygous (cir/cir) or heterozygous (+/cir) mice. There were no cells showing a severe block caused by linopirdine among the total 9 cells recorded. We succeeded in observing a 100 µM response only in one cell among the 9 cells recorded. In the remaining 8 cells, responses only up to 10 µM were observed. In 8 cells, the currents were reduced to 93.6±1.4% (n=8, 1 µM) and 90.5±2.7% (n=8, 10 µM) (Fig. 4B). In one cell, the currents at 40 mV were reduced to 94.7% (1 µM), 92.1% (10 µM), and 50.4% (100 µM) (Fig. 4A).
We also checked the effects of linopirdine on small inward currents recorded by hyperpolarizing voltage steps (from –100 mV to –70 mV). However, we did not observe any inhibition in this voltage range in all cells tested (data not shown).
Currently, only two types of K+ currents, a transiently expressed inward rectifier (IK1) [2] and a delayed rectifier-type outward (IK,neo) [112], have been reported in P0~P6 mice OHCs. A small conductance Ca2+-activated K+ current (ISK)(>P6) [34] and a K+ current activated at negative potentials (IK..n) (>P8) are the next ones expected to develop in OHCs [5]. In this report, we showed the varying degree of inhibition of K+ currents by linopirdine, an inhibitor of members of the KCNQ family K+ channels [131415], in K+ outward currents of P0~P6 heterozygous (+/cir) or homozygous (cir/cir) mice OHCs. Although the IC50s values (1~10 µM, in cases of a severe block in homozygous (cir/cir) or heterozygous (+/cir) mice) fall within the reported range of values for the inhibition of KCNQ currents [141516], we do not think it likely that the K+ outward currents have some component of IK,n which develops earlier than expected in homozygous (cir/cir) or heterozygous (+/cir) mice OHCs, as the biophysical and pharmacological properties of K+ currents in this study do not match well with those of IK,n reported in guinea-pig [17] or mice OHCs [5]. The possibility of the involvement of ISK was ruled out in the previous study with respect to TEA sensitivity [7].
Firstly, unlike IK,n reported in guinea-pig [17] or mice [5], hyperpolarizing voltage steps elicited only small inward currents without active current decay in P0~P6 heterozygous (+/cir) and homozygous (cir/cir) mice. This small inward current (less than 100 pA at –100 mV) might be influenced by a holding potential of –60 mV. However, regardless of the holding potential, IK,n was easily observable in OHCs where IK,n was fully developed [51718]. The fact that linopirdine did not inhibit inward currents recorded at hyperpolarizing voltage steps also supports the claim that IK,n is not present in heterozygous (+/cir) or homozygous (cir/cir) mice OHCs.
Secondly, the activation curves obtained from homozygous (cir/cir) and heterozygous (+/cir) mice are similar to that obtained from P6 mice OHC in which K+ outward currents activate at potentials close to –50 mV, whereas they activate at potentials negative to –100 mV in P12 mice OHCs where IK,n is already developed [5].
Thirdly, K+ outward currents in homozygous (cir/cir) or heterozygous (+/cir) mice are sensitively blocked by TEA in the millimolar range [7]. We do not know the underlying ion channel subtypes of the K+ outward currents of heterozygous (+/cir) or homozygous (cir/cir) mice OHCs, but if it is KCNQ4 as suggested by Marcotti and Kros in developing mice OHCs [5], it is not in line with our results because KCNQ4 is known to be relatively insensitive to TEA [1920]. Moreover, the fact that linopirdine did not cause any further inhibition after K+ currents were fully inhibited by TEA (data not shown) in heterozygous (+/cir) or homozygous (cir/cir) mice also supports our notion.
Marcotti and Kros reported that linopirdine up to 200 µM was not effective at all in immature mice OHCs (<P6) [5]. Up to 10 µM linopirdine, we observed similar results in ICR mice OHCs; however, in one cell, we did observe that 100 µM linopirdine inhibited K+ current at 40 mV by 50%. In hippocampal neurons, it was reported that linopirdine exceeding 100 µM inhibited the delayed rectifier K+ currents by 50% [21]. Considering the non-specific blocking effects of linopirdine on other currents [2122], K+ current inhibition by a high concentration of linopirdine might not be surprising even in immature OHCs. Species difference should be considered.
In this study, we showed the varying degree of inhibition (severe, moderate, mild) by linopirdine. This indicates that developing OHCs are not homogeneous with respect to ion channel expression. We usually used cells located in the cochlear middle turn, but not always those in the same low and the same location. It is not clear whether this inhomogeneity stems from positional difference along the organ of Corti or other unknown factors.
The purpose of this study was to investigate the ion channels contributing to OHC degeneration starting from the second postnatal week in homozygous (cir/cir) mice [10]. We do not know the exact function of this linopirdine-sensitive K+ channel in the development of homozygous (cir/cir) mice OHCs, but it can be suggested that this K+ channel does not play a significant role in OHC degeneration because a similar K+ channel is also expressed early in heterozygous (+/cir) mice OHCs.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2040535).
Notes
References
1. Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003; 548:383–400. PMID: 12588897.

2. Marcotti W, Géléoc GS, Lennan GW, Kros CJ. Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea. Pflugers Arch. 1999; 439:113–122. PMID: 10651007.

3. Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998; 10:907–915. PMID: 9753158.

4. Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci. 2011; 31:15092–15101. PMID: 22016543.

5. Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999; 520:653–660. PMID: 10545133.
6. Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998; 394:281–284. PMID: 9685158.

7. Ahn JW, Kang SW, Ahn SC. Characteristics of K+ outward currents in the cochlear outer hair cells of circling mice within the first postnatal week. Korean J Physiol Pharmacol. 2015; 19:383–388. PMID: 26170743.
8. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, Cho KI, Hong SH. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 127:244–251. PMID: 17364360.

9. Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, Lee JR, Kim HT, Suh JG, Kim TY, Ryoo ZY. Circling mouse: possible animal model for deafness. Comp Med. 2001; 51:550–554. PMID: 11924819.
10. Lee Y, Chang SY, Jung JY, Ahn SC. Reinvestigation of cochlear pathology in circling mice. Neurosci Lett. 2015; 594:30–35. PMID: 25817368.

11. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 28:13003–13007. PMID: 19036993.

12. Helyer RJ, Kennedy HJ, Davies D, Holley MC, Kros CJ. Development of outward potassium currents in inner and outer hair cells from the embryonic mouse cochlea. Audiol Neurootol. 2005; 10:22–34. PMID: 15486441.

13. Pattnaik BR, Hughes BA. Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium. Am J Physiol Cell Physiol. 2012; 302:C821–C833. PMID: 22135213.

14. Søgaard R, Ljungstrøm T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001; 280:C859–C866. PMID: 11245603.

15. Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998; 282:1890–1893. PMID: 9836639.

16. Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006; 573:17–34. PMID: 16527853.
17. Housley GD, Ashmore JF. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol. 1992; 448:73–98. PMID: 1593487.

18. Kimitsuki T, Komune N, Noda T, Takaiwa K, Ohashi M, Komune S. Property of IK,n in inner hair cells isolated from guinea-pig cochlea. Hear Res. 2010; 261:57–62. PMID: 20060884.
19. Bal M, Zhang J, Zaika O, Hernandez CC, Shapiro MS. Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J Biol Chem. 2008; 283:30668–30676. PMID: 18786918.
20. Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001; 90:1–19. PMID: 11448722.

21. Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther. 1998; 286:709–717. PMID: 9694925.
22. Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997; 9:605–616. PMID: 9104602.
Fig. 1
Whole cell K+ currents of P0~P6 mice OHCs. Current traces recorded from P6 ICR mice is shown in A.
Currents were elicited by depolarizing voltage steps from –100 mV to 40 mV with 10 mV increment (holding potential: –60 mV). Voltage protocol is shown above the current traces. Current density-voltage curve of ICR mice is shown in B. Activation curves for ICR, heterozygous (+/cir), and homozygous (cir/cir) mice are shown in C (filled square: ICR, filled circle: heterozygous (+/cir) mouse (Hetero), hollow circle: homozygous (cir/cir) mouse (Homo)). The activation curves in C were obtained by plotting the normalized tail currents at -30 mV against different prepulse potentials (–90 mV~40 mV). The voltage protocol and tail currents are shown in inset.
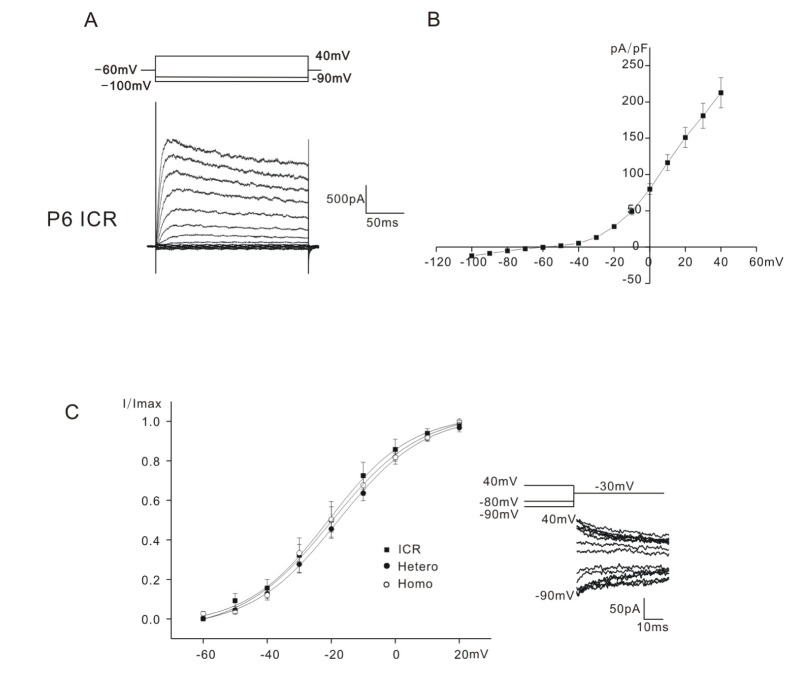
Fig. 2
Effect of linopirdine on K+ currents in homozygous (cir/cir) mice OHCs.
The varying degree of inhibition of K+ currents by linopirdine (1 to 100 µM) is shown in A, B, and C. Currents were elicited by depolarizing voltage steps from –60 mV to 40 mV (the holding potential was –60 mV). Reduced currents were normalized (I/Imax) with the peak currents at 40 mV before drug application. Normalized currents – voltage curves are shown in Ae, Be, and Ce. Marks above the current traces indicate the measuring points and linopirdine concentrations. Fig. 2C shows the linopirdine effects recorded in one cell. Fig. 2D shows the linopirdine effects recorded in one cell, currents were elicited by depolarizing voltage steps from –100 mV to 40 mV (the holding potential was –60 mV). Linopirdine was not effective at hyperpolarizing voltage range (–100 mV~–60 mV).
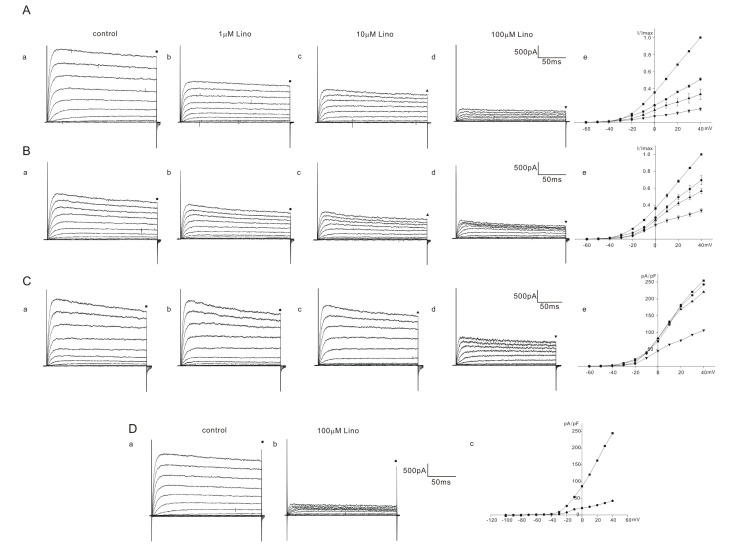
Fig. 3
Effect of linopirdine on K+ currents in heterozygous (+/cir) mice OHCs.
The varying degree of inhibition of K+ currents by linopirdine is shown in A (1 to 100 µM) and B (1 to 10 µM). Currents were elicited by depolarizing voltage steps from –60 mV to 40 mV (the holding potential was –60 mV). Reduced currents were normalized (I/Imax) with peak currents at 40 mV before drug application. Normalized currents–voltage curves are shown in Ae and Bd. Marks above the current traces indicate the measuring points and linopirdine concentrations.
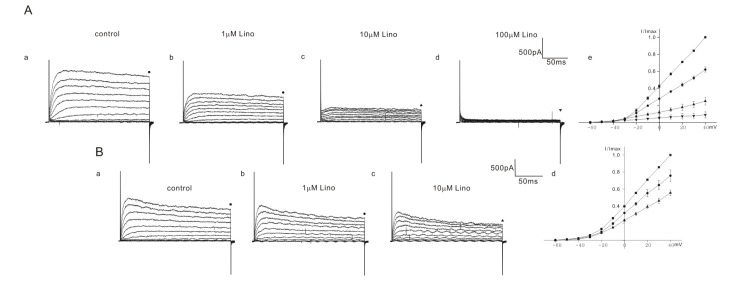
Fig. 4
Effect of linopirdine on K+ currents in ICR mice OHCs.
The varying degree of inhibition of K+ currents by linopirdine is shown in A (1 to 100 µM) and B (1 to 10 µM). Currents were elicited by depolarizing voltage steps from –60 mV to 40 mV (the holding potential was –60 mV). Reduced currents were normalized (I/Imax) with peak currents at 40 mV before drug application. Normalized currents-voltage curves are shown in Ae and B. Marks above the current traces indicate the measuring points and linopirdine concentrations. Fig. 4Aa~4Ae show the linopirdine effects recorded in one cell. Fig. 4B shows normalized currents-voltage curves obtained from the cells treated with linopirdine up to 10 µM.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download