Abstract
Understanding the crosstalk mechanisms between perivascular cells (PVCs) and cancer cells might be beneficial in preventing cancer development and metastasis. In this study, we investigated the paracrine influence of PVCs derived from human umbilical cords on the proliferation of lung adenocarcinoma epithelial cells (A549) and erythroleukemia cells (TF-1α and K562) in vitro using Transwell® co-culture systems. PVCs promoted the proliferation of A549 cells without inducing morphological changes, but had no effect on the proliferation of TF-1α and K562 cells. To identify the factors secreted from PVCs, conditioned media harvested from PVC cultures were analyzed by antibody arrays. We identified a set of cytokines, including persephin (PSPN), a neurotrophic factor, and a key regulator of oral squamous cell carcinoma progression. Supplementation with PSPN significantly increased the proliferation of A549 cells. These results suggested that PVCs produced a differential effect on the proliferation of cancer cells in a cell-type dependent manner. Further, secretome analyses of PVCs and the elucidation of the molecular mechanisms could facilitate the discovery of therapeutic target(s) for lung cancer.
The tumor microenvironment (TME) is the noncancerous cellular region in which tumors exist [1]. The TME is composed of the extracellular matrix and various cell types, including endothelial cells (ECs), fibroblasts, macrophages, and perivascular cells (PVCs) [12]. These cells are involved in regulating tumor growth, invasion, angiogenesis, and metastasis, both through direct cell-to-cell contact and by a paracrine signaling mechanism [3]. Therefore, understanding the communication between the tumor and TME is critical to identifying therapeutic agent(s) for the prevention of tumor growth and metastasis [13].
PVCs are enclosed within the basement membrane of blood vessels. Crisan et al. and others [456] have reported that PVCs are ancestors of multipotent mesenchymal stem cells (MSCs) in multiple tissues of both fetal and adult origin, and have greater regenerative potential compared to MSCs. The use of PVCs has increasingly gained attention as an alternative therapeutic candidate for the treatment of various diseases [456]. In normal and developmental contexts, PVCs contribute to tissue homeostasis and repair by regulating vascular stability and contractility [3]. PVCs are also key components of the TME, and are therefore able to induce the onset of angiogenesis and apoptosis through crosstalk with ECs in pathological conditions such as cancer and hyperglycemia [1].
For example, in tumors, angiopoietin-2 (ANG2) is primarily secreted from activated ECs to destabilize blood vessels by inducing PVC detachment and EC sprouting via the antagonizing factor ANG1 [789]. In ECs exposed to high glucose, miR-503 transcriptionally activated and modulated p75NTR, affecting the migration and proliferation of PVCs via the reduction of ephrin-B (EFNB2) and vascular endothelial growth factor A (VEGFA) expression [10]. Therefore, the modulation of PVC-EC interactions could provide a therapeutic strategy to inhibit cancer growth and/or manage diabetes complications.
Although the underlying molecular mechanisms of the angiogenic contribution of PVCs are relatively well defined, the effects and molecular mechanisms of PVCs on the proliferation of cancer cells are still unclear. Paracrine signaling pathways between PVCs and cancer cells have been suggested as an important mechanism in tumor growth and metastasis [1]. Therefore, the identification of key secretory factors that regulate the proliferation of cancer could provide valuable insight into the prevention and development of tumors. In the present study, we isolated PVCs from human umbilical cords (HUCs) and investigated their paracrine influences on the proliferation of human lung adenocarcinoma (A549) and erythroleukemia cell (TF-1α and K562) lines in vitro using Transwell® co-culture systems. PVCs promoted the proliferation of lung adenocarcinoma cells, but not erythroleukemia cells, which was mediated by the release of soluble factors from the PVCs.
HUC tissues were obtained from full term births after Caesarian section with informed consent using the guidelines approved by IRB (IRB approval number: KNUH-2012-11-003-008) at the Kangwon National University Hospital. PVCs were isolated and cultured as previously described [11]. A549, K-562, and TF-1α cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). A549 cells were grown in Dulbecco's Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). K562 cells were grown in Iscove's Modified Dulbecco's Media (IMDM; Hyclone) supplemented with 10% FBS and 1% penicillin/streptomycin. TF-1α cells were cultured in RPMI (Hyclone) supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were cultured at 37℃ and 5% CO2 and subcultured at 80~90% confluency.
The phenotypes of PVCs were analyzed by flow cytometry as previously described [12]. Briefly, single cell suspensions (passage 2) were incubated with CD31-phycoerythrin, CD34-fluorescein isothiocyanate (FITC), CD45-allophycoerythrin (APC), CD44-APC, CD90-APC, and CD146-FITC for 60 minutes at 4℃ in the dark. The cells were rinsed with 1% FBS-phosphate-buffered saline (PBS) and analyzed on a BD FACSCanto™ II flow cytometer (BD Biosciences, San Jose, CA, USA). Dead cells were excluded by staining with 7AAD viability staining solution (BD Biosciences). Acquired data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). All antibodies were purchased from BD Biosciences.
The multilineage differentiation potentials of the PVCs were evaluated as previously described [13]. Briefly, PVCs from passage 3 were seeded at 4×104 cells/well in expansion medium. When the cells reached 80% confluency, they were treated with osteogenic or adipogenic induction medium (StemPro®, ThermoFisher Scientific, San Jose, CA, USA) for 21 days at which time the medium was changed every 3 days. PVC cultures were stained with Alizarin Red S (CM-0058; Lifeline Cell Technology, Carlsbad, CA, USA) or Oil Red O (CM-0055; Lifeline Cell Technology) and the dye contents were quantified using a spectrophotometer.
Transwell® plates (Corning, Corning, NY, USA) with 0.4 µm pore polycarbonate membrane inserts were used for the co-culture experiments. A549 cells (7×104) were co-cultured for 48 hrs with PVCs at a ratio of 1:1 and 1:3 (A549:PVCs). TF-1α and K562 cells (2×105 and 3×105, respectively) were also co-cultured for 48 hrs with PVCs at a ratio of 1:1 and 1:3 (TF-1α or K562:PVCs, respectively). PVCs were seeded into the lower chamber, and cancer cells were placed in the upper chamber. Cell number and size were measured using the MOXI Z automated cell counter kit (ORFLO Technologies, Carlsbad, CA, USA).
Antibody arrays (Human L507 array kit, AAH-BLG-1; Ray-Biotech, Norcross, GA, USA) were used to profile the secretions from PVCs. When PVCs reached 90% confluency, the cells were cultured in defined serum-free medium for 24 hrs to condition the growth medium. The resulting conditioned medium (CM) was harvested, centrifuged, and filtered using 0.2 µm filters to remove any cell debris. The CM was concentrated using an Amicon Ultra-0.5 centrifugal filter unit with a cutoff of 3 kDa (EMD Millipore, Hayward, CA, USA), and the concentrated CM was stored at −80℃. Non-CM was used as a control. The concentrated medium was used for the antibody array analyses according to the manufacturer's instructions. Secretion factors that were 1.5-fold or greater relative to the control were scored as significant.
To determine the effect of PSPN on the proliferation of A549 cells, A549 cells were seeded at a density of 2×105 cells into 6-well culture plates and cultured in the presence of PSPN (10, 20, and 40 ng/ml) for 48 hrs. In experiment blocking PSPN signals, anti-PSPN antibody (MyBioSource, San Diego, CA, USA) at a final concentration of 500 ng per mL was added in A549 cultures. Cell number and morphology were evaluated using the MOXI Z automated cell counter (ORFLOW Technologies).
Total RNA from cancer cell lines was extracted with the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) and converted to first strand cDNA with TOPscript™ RT DryMIX kit (Enzynomix). The cDNA of GFRα4 mRNA was amplified and quantified using AccuPower® PCR PreMix reagents (Bioneer Corp). Primer sequence was as follow: GFRα4, forwa rd 5'-AGGAGGCATCTTGGTTGTAA-3' and reverse 5'-GGGTCTCCAGAGAGGTCTCA-3', GAPDH, forward 5'-TGCACCACCAACTGCTTAGC-3' and reverse 5'-GGCATGGACTGTGGTCATGAG-3'. The expression level of GFRα4 was normalized to GAPDH and data analysis was performed by the comparative CT method.
PVCs were isolated and cultured from vessels of umbilical cords by a non-enzymatic isolation method [11]. Isolated PVCs displayed spindle-shaped, fibroblast-like morphology as seen in MSCs (Fig. 1A). PVCs were phenotypically identified as strongly positive for the expression of CD44 (99.8%, MSC marker), CD90 (100%, MSC marker), and CD146 (87.2%, PVC marker) and as negative for the CD34 (hematopoietic progenitor marker), CD31 (endothelial cell marker), and CD45 (mature hematopoietic cell marker) (Fig. 1B). In addition, we confirmed that the PVCs had multilineage differentiation potentials, including for adipocytes, osteocytes, and chondrocytes (Fig. 1C and 1D). These results indicated that the isolated and expanded cells consisted of PVCs.
To determine the effects of PVCs on the proliferation of cancer cells, PVCs were indirectly co-cultured with human lung adenocarcinoma cells (A549) and erythroleukemia cells (TF-1α and K562) using Transwell® co-culture systems. We assumed that PVCs can differentially regulate the growth of adherent (A549) and non-adherent (TF-1α and K562) cancer cells. Cancer cell lines were co-cultured with PVCs at a ratio of 1:1 and 1:3 for 48 hrs. The proliferation of all cancer cells was not affected by co-culture at a ratio of 1:1. The proliferation of TF-1α and K562 cells was also not affected by co-culture at a ratio of 1:3. However, the number of A549 cells increased approximately two-fold after indirect co-culturing with PVCs at a ratio of 1:3 (Fig. 2A). Cell morphology and diameter of all cancer cell lines were not altered by co-culturing with PVCs at both ratios (Fig. 2B and 2C). These findings suggested that PVCs had differential effects on the proliferation of cancer cells in a cell-type dependent manner, and they promoted the proliferation of human lung adenocarcinoma cells via paracrine actions.
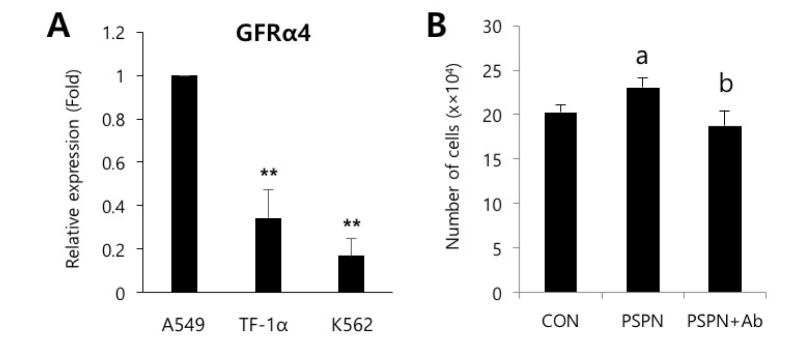
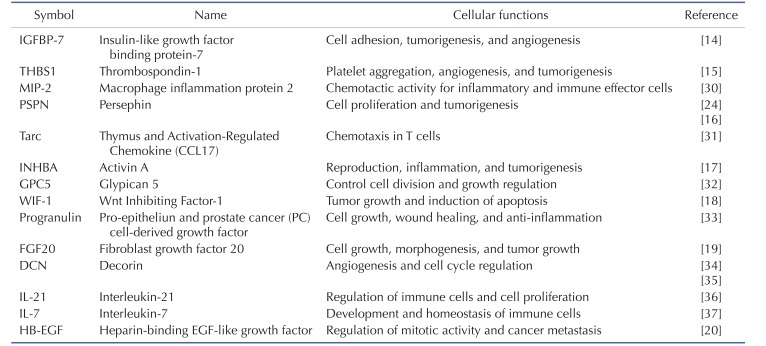
To identify the secreted factors, the PVC-CM and non-CM were analyzed using 507 antibody array chips for secreted soluble factors. Secreted factors that had 1.5-fold or more expression relative to the non-CM controls were considered as significantly abundant. We found that 14 cytokines and growth factors were significantly abundant in the PVC-CM compared with the non-CM controls (Fig. 3A). Some cellular functions of these factors are shown in Table 1. Some cytokines and growth factors, such as IGFBP-7, THBS1, PSPN, INHBA, WIF-1, FGF20, and HB-EGF play important roles in regulating tumor growth and metastasis [14151617181920]. Of these identified factors, we further investigated the effects of PSPN on the proliferation of A549 cells because its effects on tumorigenesis are less understood. A549 cells were treated with different concentrations of PSPN (10, 20, and 40 ng/ml) for 48 hrs. Supplementation with 20 ng/ml or 40 ng/ml of PSPN significantly promoted the proliferation of A549 cells without inducing morphological changes (Fig. 3B and 3C). These results indicated that the growth of lung adenocarcinoma cells was influenced by PVCs and by PSPN via paracrine signaling. To determine if the paracrine effect of PSPN on A549 proliferation is mediated via its receptor signaling pathway, we compared expression level of GFRα4 in A549, TF-1α and K562 cell cultures. We found that GFRα4 transcripts was significantly more abundant in A549 cells than TF-1α and K562 cells (Fig. 4A). We also confirmed the suppressive effect of a neutralizing anti-PSPN antibody on the proliferation of A549 cells (Fig. 4B), suggesting that PSPN promotes proliferation of human adenocarcinoma cells by its receptor-mediated pathway.
In the present study, we performed secretome analyses of PVC-CM and identified a set of secreted cytokines known to be important in regulating the cell cycle, tumorigenesis, angiogenesis, and inflammation. The most abundant cytokine in PVC-CM compared with non-CM was IGFBP-7, which is linked to the suppression of growth and metastasis of tumors. For example, the activation of the IGFBP-7 gene suppresses the invasion of head and neck squamous cell carcinoma and acute myeloid leukemia (AML) cells [2122]. These studies suggest that IGFBP-7 could be a prognostic factor for the probability of invasion as well as a therapeutic target for the improvement of AML patient survival. THBS1 and PSPN are also highly detected in the PVC-CM. THBS1, a glycoprotein, is implicated in the regulation of tumor growth and metastasis [1523]. PSPN was first identified as a glial cell line-derived neurotrophic factor (GDNF) that had neurotrophic effects on some neuronal cell types. For example, PSPN enhanced the morphological differentiation and phenotypic induction of dopaminergic neurons and could be considered as a cell therapy for Parkinson's disease [24252627]. However, very little is known about the role of PSPN in tumor progression. To the best of our knowledge, only one study has explored the effects of PSPN on the proliferation of cancer. Baba et al. reported that PSPN was a key factor in proliferation of human oral cancer through activation of RET-mitogen-activated protein kinase [16]. Our study showed that, while supplementation of PSPN promoted the proliferation of human lung adenocarcinoma cells, it did not affect the proliferation of erythroleukemia cells, because PVC co-culturing did not change the growth of TF-1α and K562 cells. These results suggested a differential effect of PVCs on the proliferation of cancer cells in a cell-type dependent manner. Thus, an investigation of PSPN in a broader range of tumor cell types should be done.
Although multipotent MSCs for use in clinical trials have been shown to be safer and more effective than pluripotent stem cells, their roles in cancer development remain controversial. This could be attributed to the different MSC sources, variations in donors, and the injection timing and dosage of MSCs [28]. Because PVCs have been proposed as common ancestors of MSCs, they could provide an alternative source due to their greater regeneration potential compared with other MSCs [456]. Thus, the role of PVCs in cancer development should be addressed prior to clinical applications, especially for patients with cancer who are undergoing or have undergone treatment. Sinha et al. reported that co-culturing with PVCs increased the proliferation of ovarian cancer cells in vitro [29]. Similarly, our results also showed that the proliferation of lung adenocarcinoma cells was increased by co-culturing with PVCs, and this effect was mediated by secreted factors from the PVCs. Thus, identification of secreted factors and their functional evaluation related with tumor growth could be critical to the discovery of novel anticancer therapy.
In conclusion, PVCs significantly contributed to lung cancer growth, and this could have been by paracrine signaling. Further study is required using in vivo animal models and direct injections of PVC-CM or selected paracrine factors to define the role of PVCs in lung cancer growth and metastasis.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014-R1A1A2055021), the Ministry of Science, ICT & Future Planning (2015R1A4A1038666) and Kangwon National University.
Notes
Author contributions: E.B.K., S.H.N., B.R.A., S.R.Y., J.Y.L. and W.J.K. contributed to the study design, performed the statistical analyses, contributed to the interpretation of data, and drafted the manuscript; K.W.H., W.S.P., C.M.L., and E.T.H. contributed to the acquisition of the data and to the critical review of the manuscript; S.J.L. and S.H.H. contributed to the study design, to the interpretation of the data, and to the critical review of the manuscript. All authors read and approved the final manuscript.
References
1. Ribeiro AL, Okamoto OK. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015; DOI: 10.1155/2015/868475.

2. Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006; 34:763–775. PMID: 17162534.

4. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008; 3:301–313. PMID: 18786417.

5. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007; 25:1384–1392. PMID: 17332507.

6. Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010; 6:197–203. PMID: 21220956.
7. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009; 10:165–177. PMID: 19234476.

8. Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005; 118:771–780. PMID: 15687104.

9. Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009; 12:125–137. PMID: 19449109.

10. Caporali A, Meloni M, Nailor A, Mitić T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, Voellenkle C, Verkade P, Martelli F, Madeddu P, Emanueli C. p75(NTR)-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015; 6:8024. PMID: 26268439.

11. An B, Na S, Lee S, Kim WJ, Yang SR, Woo HM, Kook S, Hong Y, Song H, Hong SH. Non-enzymatic isolation followed by supplementation of basic fibroblast growth factor improves proliferation, clonogenic capacity and SSEA-4 expression of perivascular cells from human umbilical cord. Cell Tissue Res. 2015; 359:767–777. PMID: 25501896.

12. Hong SH, Maghen L, Kenigsberg S, Teichert AM, Rammeloo AW, Shlush E, Szaraz P, Pereira S, Lulat A, Xiao R, Yie SM, Gauthier-Fisher A, Librach CL. Ontogeny of human umbilical cord perivascular cells: molecular and fate potential changes during gestation. Stem Cells Dev. 2013; 22:2425–2439. PMID: 23557155.

13. An B, Heo HR, Lee S, Park JA, Kim KS, Yang J, Hong SH. Supplementation of growth differentiation factor-5 increases proliferation and size of chondrogenic pellets of human umbilical cord-derived perivascular stem cells. Tissue Eng Regen Med. 2015; 12:181–187.

14. Kashyap MK. Role of insulin-like growth factor-binding proteins in the pathophysiology and tumorigenesis of gastroesophageal cancers. Tumour Biol. 2015; 36:8247–8257. PMID: 26369544.

15. Sid B, Sartelet H, Bellon G, El Btaouri H, Rath G, Delorme N, Haye B, Martiny L. Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor growth. Crit Rev Oncol Hematol. 2004; 49:245–258. PMID: 15036264.

16. Baba T, Sakamoto Y, Kasamatsu A, Minakawa Y, Yokota S, Higo M, Yokoe H, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Persephin: A potential key component in human oral cancer progression through the RET receptor tyrosine kinase-mitogen-activated protein kinase signaling pathway. Mol Carcinog. 2015; 54:608–617. PMID: 24375483.

17. Wijayarathna R, de Kretser DM. Activins in reproductive biology and beyond. Hum Reprod Update. 2016; 22.

18. Huang Y, Du Q, Wu W, She F, Chen Y. Rescued expression of WIF-1 in gallbladder cancer inhibits tumor growth and induces tumor cell apoptosis with altered expression of proteins. Mol Med Rep. 2016; 14:2573–2581. PMID: 27430608.

19. Koga C, Adati N, Nakata K, Mikoshiba K, Furuhata Y, Sato S, Tei H, Sakaki Y, Kurokawa T, Shiokawa K, Yokoyama KK. Characterization of a novel member of the FGF family, XFGF-20, in Xenopus laevis. Biochem Biophys Res Commun. 1999; 261:756–765. PMID: 10441498.

20. Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997; 1333:F179–F199. PMID: 9426203.

21. Chen LH, Liu DW, Chang JL, Chen PR, Hsu LP, Lin HY, Chou YF, Lee CF, Yang MC, Wen YH, Hsu WL, Weng CF. Methylation status of insulin-like growth factor-binding protein 7 concurs with the malignance of oral tongue cancer. J Exp Clin Cancer Res. 2015; 34:20. PMID: 25880247.

22. Verhagen HJ, de Leeuw DC, Roemer MG, Denkers F, Pouwels W, Rutten A, Celie PH, Ossenkoppele GJ, Schuurhuis GJ, Smit L. IGFBP7 induces apoptosis of acute myeloid leukemia cells and synergizes with chemotherapy in suppression of leukemia cell survival. Cell Death Dis. 2014; 5:e1300. PMID: 24967962.

23. Teraoku H, Morine Y, Ikemoto T, Saito Y, Yamada S, Yoshikawa M, Takasu C, Higashijima J, Imura S, Shimada M. Role of thrombospondin-1 expression in colorectal liver metastasis and its molecular mechanism. J Hepatobiliary Pancreat Sci. 2016; 23:565–573. PMID: 27404020.

24. Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Johnson EM Jr. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998; 20:245–253. PMID: 9491986.

25. Zihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR. The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull. 2005; 68:42–53. PMID: 16325003.

26. Roussa E, Oehlke O, Rahhal B, Heermann S, Heidrich S, Wiehle M, Krieglstein K. Transforming growth factor beta cooperates with persephin for dopaminergic phenotype induction. Stem Cells. 2008; 26:1683–1694. PMID: 18420832.
27. Murata T, Tsuboi M, Koide N, Hikita K, Kohno S, Kaneda N. Neuronal differentiation elicited by glial cell line-derived neurotrophic factor and ciliary neurotrophic factor in adrenal chromaffin cell line tsAM5D immortalized with temperature-sensitive SV40 T-antigen. J Neurosci Res. 2008; 86:1694–1710. PMID: 18293415.

28. Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Front Genet. 2013; 4:261. PMID: 24348516.

29. Sinha D, Chong L, George J, Schlüter H, Mönchgesang S, Mills S, Li J, Parish C, Bowtell D, Kaur P. Australian Ovarian Cancer Study Group. Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin Cancer Res. 2016; 22:1813–1824. PMID: 26589433.

30. Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994; 20:473–490. PMID: 7882902.

31. Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997; 272:15036–15042. PMID: 9169480.

32. Li F, Shi W, Capurro M, Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol. 2011; 192:691–704. PMID: 21339334.

33. Nicoletto BB, Canani LH. The role of progranulin in diabetes and kidney disease. Diabetol Metab Syndr. 2015; 7:117. PMID: 26697121.

34. Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol. 2015; 43:15–26. PMID: 25661523.

35. Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013; 110:E2582–E2591. PMID: 23798385.

36. Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000; 408:57–63. PMID: 11081504.

37. Muegge K, Vila MP, Durum SK. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993; 261:93–95. PMID: 7686307.

Fig. 1
Isolation and characterization of PVCs derived from HUCs.
(A) The morphology of the PVCs in culture. Fibroblast-like colonies were subcultured and expanded. Bars=100 µm. (B) Representative flow cytometry histograms for phenotypes of PVCs (passage 2) are shown. The pink histogram indicates the isotype control. (C) Evaluation of multilineage differentiation potentials of the PVCs. Representative images of Oil Red O staining for adipocytes, Alizarin Red S staining for osteocytes, and Alcian Blue staining for chondrocytes are shown. Bars=100 µm, or 500 µm. (D) Quantification of the dye (Oil Red O and Alizarin Red S) content using spectrophotometry. PVCs, perivascular cells; HUCs, human umbilical cords; CON, control cultures; EXP, experimental cultures with differentiation conditions.
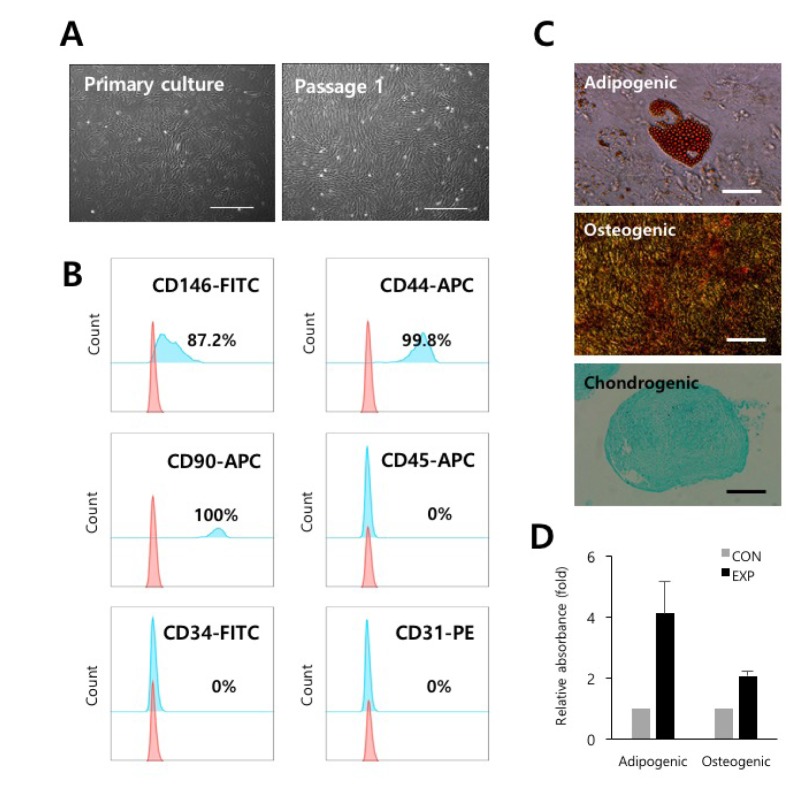
Fig. 2
PVCs significantly increase the proliferation of human lung adenocarcinoma cells.
(A) Indirect co-culturing of PVCs with human erythroleukemia cell lines (TF-1α and K562) and lung adenocarcinoma cell line (A549). The error bars indicate the standard deviation. **p<0.01. (B) The morphology of the cancer cells co-cultured with PVCs for 48 hrs. Bars=50 µm. (C) The diameter of the cancer cells was measured after co-culturing with PVCs for 48 hrs. The error bars indicate the standard deviation. CON, control group; PVCs, perivascular cells.
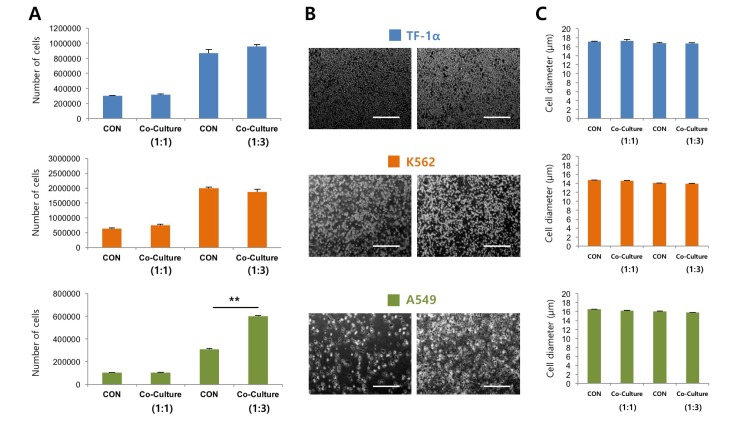
Fig. 3
PSPN contributes to the proliferation of human lung adenocarcinoma cells.
(A) Secretome analyses of PVC-CM using antibody arrays. A list of selected cytokines that had 1.5 fold or more expression relative to the control sample (non-CM) is shown. (B) A549 cells were plated at a density of 2×105 cells and treated with a variety of concentrations of PSPN (10, 20, and 40 ng/ml) for 48 hrs. The error bars indicate standard deviation. *p<0.05, **p<0.01. (C) Representative images of A549 cells cultured in the presence and absence of PSPN for 48 hrs. Bars=50 µm. CON, control group; PSPN, Persephin; PCV-CM, perivascular cell-conditioned medium; non-CM, non-conditioned control medium.
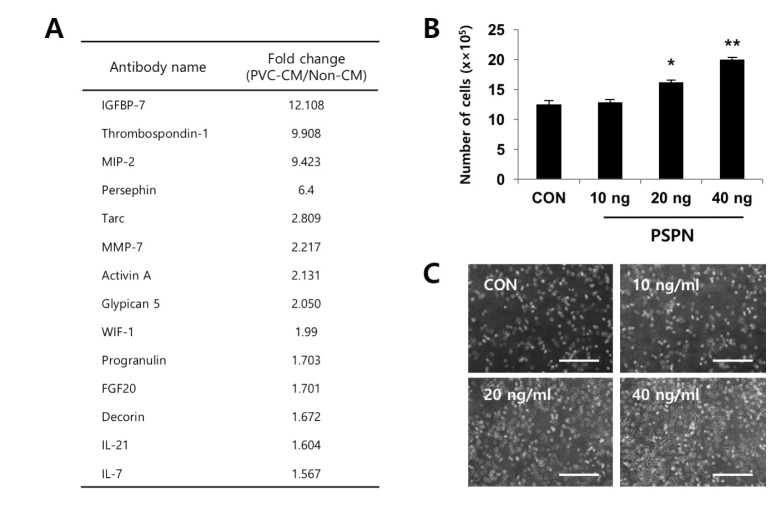
Fig. 4
Neutralization of PSPN inhibits proliferation of human lung adenocarcinoma cells.
(A) qPCR analysis of relative GFRα4 expression in A549, TF-1α and K562 cells. (B) PSPN neutralizing antibody (500 ng/mL) suppressed the increased proliferation of A549 cells by PSPN (40 ng/mL) treatment. The error bars indicate the standard deviation. **p<0.01, a, p<0.01. (control vs PSPN), b, p<0.01. (PSPN vs PSPN+Ab).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download