Abstract
Conflicting evidence has been obtained regarding whether transient receptor potential cation channels (TRPC) are store-operated channels (SOCs) or receptor-operated channels (ROCs). Moreover, the Ca/Na permeability ratio differs depending on whether the current-voltage (I-V) curve has a doubly rectifying shape or inward rectifying shape. To investigate the calcium permeability of TRPC4 channels, we attached GCaMP6s to TRPC4 and simultaneously measured the current and calcium signals. A TRPC4 specific activator, (–)-englerin A, induced both current and calcium fluorescence with the similar time course. Muscarinic receptor stimulator, carbachol, also induced both current and calcium fluorescence with the similar time course. By forming heteromers with TRPC4, TRPC1 significantly reduced the inward current with outward rectifying I-V curve, which also caused the decrease of calcium fluorescence intensity. These results suggest that GCaMP6s attached to TRPC4 can detect slight calcium changes near TRPC4 channels. Consequently, TRPC4-GCaMP6s can be a useful tool for testing the calcium permeability of TRPC4 channels.
The transient receptor potential (TRP) channel was initially cloned in fruit flies [1]. The TRP channel is activated by rhodopsin, a G protein-coupled receptor (GPCR), and responds to light. Since the initial reports, GPCR-activated TRP channels were subsequently cloned in mammalian tissues and named classical type TRP (TRPC) channels. The TRPC subfamily consists of seven known members [2]. Some studies have shown that TRPCs 1, 3, 4, and 5 can be activated by depletion of endoplasmic reticulum Ca2+ stores, suggesting that these channels contribute to SOC entry. On the contrary, other studies have proposed that these homologues contribute to ROC entry [3]. In the studies that focused specifically on endogenous TRPC proteins contributing to SOC entry in endothelial cells, TRPC1 and TRPC4 were shown to contribute to the endothelial Ca2+-selective ISOC channel. Previously, antisense technology was used to assess the contribution of endogenous TRPC1 to ISOC in pulmonary artery endothelial cells (PAECs) [4]. Inhibition of TRPC1 reduced but did not eliminate ISOC. On the other hand, endothelial cells obtained from TRPC4-deficient mice completely lacked ISOC [5]. These studies suggest that, although TRPC1 and TRPC4 both contribute subunits to the ISOC channel, only TRPC4 is required for channel activation.
TRP channels are calcium-permeable cation channels. As a family member of TRP channel, TRPC channels are nonselective cation channels permeable to calcium as well as monovalent cations. When calcium permeability is calculated with shift of reversal potentials, the permeability of these channels to monovalent cations ranges from 1 to 7 [678]. On the other hand, TRPC4 [a component of the Ca2+ release-activated Ca2+ (CRAC) channel] has been calculated to have a higher ratio of 159.7 [5]. The permeability ratio has been shown to differ depending on whether the I-V curve has a doubly rectifying shape or an inward rectifying shape.
In gastrointestinal smooth muscle, acetylcholine binds to the muscarinic receptor and induces calcium release from calcium stores through the inositol triphosphate (IP3) receptor [9]. In addition, acetylcholine activates nonselective cation channels and induces membrane depolarization, which in turn activates the voltage-operated calcium channel (VOCC). The mobilization of calcium through the VOCC increases the intracellular calcium. TRPC4 channels are candidate nonselective cation channels that are activated by muscarinic stimulation and contribute to membrane depolarization rather than to intracellular calcium increase [10].
Calcium ions modulate the TRPC5 channel through direct action, calmodulin [1112], and myosin light chain kinase (MLCK) [1314]. TRPC4 belongs to the same subfamily as TRPC5. Schaefer et al. [8] also observed critical dependence of TRPC4 and TRPC5 activity on [Ca2+]i. Calcium influx through the TRPC4 channel might modulate TRPC channels near the channel itself as a feedback mechanism [15].
GCaMP is one of the genetically encoded calcium indicators (GECIs) which is a powerful tool for tracking the Ca2+ dynamics in target cells. GCaMP is composed of green fluorescent protein (GFP), calmodulin (CaM) and CaM-interacting M13 peptide. Ca2+ binding to the CaM result in a conformational change in CaM-M13 complex, eliminates bright fluorescence. Previously, a GCaMP3-tagged TRPML channel was used to measure lysosomal calcium release [16]. GCaMP3-TRPML1 responded to calcium released from the lysosome, but not to calcium released from the endoplasmic reticulum (ER), indicating the existence of calcium nanodomains near the lysosome. To measure the calcium concentrations near specific channels, we tagged channels at their C-terminus with GCaMP6s. Using this construct, we investigated the calcium permeability of the TRPC4 channel, the role of calcium in TRPC4 function, and the role of TRPC1 in the calcium permeability of TRPC4 channels.
Human embryonic kidney (HEK293) cells were incubated in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated FBS and penicillin (100 units/ml), streptomycin (100 µg/ml) at 37℃ in 5% CO2 humidified incubator. The mTRPC4β-GCaMP6s construct was made by inserting the full-length GCaMP6s sequence between the AgeI and NotI site of a pEYFP-N1 plasmid which contains the mouse TRPC4β complementary DNA. All constructs were confirmed by sequencing. Every plasmid DNA was prepared using a plasmid midi kit (QIAGEN) as detailed in the protocol. For the transfection, the cells were seeded in 12-well plates. The following day, mTRPC4β-GCaMP6s and other genetically encoded calcium indicators (GECI) were also transfected for electrophysiology and Ca2+ imaging by using the transfection reagent FuGENE 6 (Roche Molecular Biochemicals), as detailed in the manufacture's protocol. We co-expressed mTRPC4β-GCaMP6s and type 2 muscarinic (M2) receptor by 1:1, mTRPC4β-GCaMP6s and hTRPC1α by 2:1 ratio.
The patch pipette containing standard intracellular solution; 140 mM CsCl, 10 mM HEPES, 0.5 mM EGTA, 3 mM Mg-ATP, 0.2 mM Tris-GTP, PH 7.3 with CsOH. External solution was perfused constantly as follows; 3.6 mM KCl, 10 mM HEPES, 1 mM MgCl2, 145 mM NaCl, 2 mM CaCl2, 5 mM glucose, PH 7.4 with NaOH. We used TRPC4 channel agonist, (–)-englerin A (EA, Sigma-Aldrich) and muscarinic receptor agonist, carbachol (CCh, Sigma-Aldrich). To identify the only effect of CCh to TRPC4 channel, we changed solution from normal tyrode (NT) to thapsigargin (TG, Sigma-Aldrich) first to block the ability to pump calcium into the ER. We used Ca2+ ionophore, ionomycin (IM, Sigma-Aldrich) to raise the intracellular calcium level.
The cells were transferred onto a small chamber on the stage of an inverted microscope (I×70, Olympus, Japan) and attached to coverslip in the small chamber for 10 min prior for the patch recording. Glass microelectrodes with 2~2.5 megaohms resistance were used to obtain gigaohm seals. The currents were recorded using an Axopatch 200B patch-clamp amplifier (Axon instrument, USA). The whole cell configuration was used to measure the TRPC4 channel current in the HEK293 cells. Voltage ramps ranging from +100 to –100 mV over period of 500 msec were imposed every 10 sec with a holding membrane potential of –60 mV. pCLAMP software (version 10.2, Axon instrument, USA) were used for data acquisition and the data were analyzed using the OriginPro 8 (OriginLab, USA). The recording chamber was continuously perfused at a flow rate of 1~2 ml/min.
To obtain the image to measure calcium influx, we used an inverted microscope (I×70, Olympus, Japan) with 60× oil objective lens. Each image was captured on EMCCD camera (iXon3, Andor, Northern Ireland) every 2 sec and the fluorescence intensity at 500 nm (F500) was monitored using the MetaMorph7.6 software (Molecular devices, USA) system. Whole cell current and calcium imaging were recorded simultaneously in almost all experiments.
The FRET ratio (FR) is equal to the fractional increase in YFP emission due to FRET and was calculated as FR=FAD
AD/FA=[SFRET(DA)–RD1·SCFP (DA)]/(RA1·[SYFP (DA)–RD2·SCFP (DA)]). Here, SCUBE(SPECIMENDA) denotes an intensity measurement, where CUBE indicates the filter cube (CFP, YFP, or FRET), and SPECIMEN indicates whether the cell is expressing the donor (D; CFP), acceptor (A; YFP), or both (DA). RD1=SFRET(D)/SCFP(D), RD2=SYFP(D)/SCFP(D), and RA1=SFRET(A)/SYFP(A) are predetermined constants from measurements applied to single cells expressing only CFP- or YFP-tagged molecules. Although three-cube FRET does not require that CFP and YFP fusion constructs preserve the spectral features of the unattached fluorophores, similar ratios and recorded spectra furnished two indications that the spectral features of the fluorophores were largely unperturbed by fusion. Since the FR relies on YFP emission, YFP should be attached to the presumed limiting moiety in a given interaction. Subsequent quantitative calculations based on FR relied on a presumed 1:1 interaction stoichiometry. The effective FRET efficiency (EEFF) was determined by EEFF=E·Ab=(FR–1)·[EYFP(440)/ECFP(440)], where E is the intrinsic FRET efficiency when fluorophore-tagged molecules are associated with each other, Ab is the fraction of YFP-tagged molecules that are associated with CFP-tagged molecules, and the bracketed term is the ratio of YFP and CFP molar extinction coefficients scaled for the FRET cube excitation filter [18]. We determined this ratio to be 0.094 based on maximal extinction coefficients for ECFP and EYFP [19] and excitation spectra.
Three GECIs were used to detect calcium changes, GCaMP6s, R-GECO, and Yellow Cameleon 6.1 (YC 6.1) (Fig. 1). Depending on Kd value, the GECIS exhibited different responses to changes in extracellular calcium and ionomycin (IM, 10 µM) (Fig. 1A). GCaMP6s responded to a change in external calcium from 2 mM to 0 mM (Fig. 1B). On the other hand, R-GECO did not respond to this change (Fig. 1C). YC6.1 has a similar Kd value to GCaMP6s, and responded to calcium addition induced by IM, but with slower kinetics than GCaMP6s (Fig. 1D). Based on these results, we tagged the C-terminus of the mTRPC4β channel with GCaMP6s.
We first measured current and calcium signal simultaneously and compared their values with those from cells expressing both mTRPC4β and GCaMP6s (Fig. 2). Englerin A (EA, 100 nM) [2021], a new agonist of TRPC4/5 channels, induced a large current but did not increase the calcium signal (Fig. 2A). On the other hand, IM did not induce any change in current but increased the calcium signal. A previous report concluded that TRPC4 is an SOC and contributes to intracellular calcium increase [22]; our data contradict this finding. Our results suggest that cytosolic GCaMP6s do not detect the calcium change induced by mTRPC4β activation, and that mTRPC4β does not contribute a significant amount of calcium to the cytosol. We next attached GCaMP6s to the C-terminus of mTRPC4β, thereby generating an mTRPC4β-GCaMP6s construct, and tested whether this GCaMP6s tag could detect calcium influx through TRPC4 channels (Fig. 2B). Englerin A induced a large current in mTRPC4β-GCaMP6s, similar to its action on TRPC4 channels (Fig. 2C). The I-V curve showed a doubly rectifying shape. The measured calcium signal is shown in Fig. 2D. EA increased the fluorescence intensity of GCaMP6s attached to mTRPC4β. In contrast to free GCaMP6s, GCaMP6s attached to TRPC4 channels could detect calcium changes near the channels. We next measured the current and calcium signals simultaneously (Fig. 2E). EA increased both the current and the calcium fluorescence; these values returned to those of the control after washout of EA. In contrast, IM increased the calcium fluorescence but did not increase the current. The time to the peak current (77.2±6.8 sec, n=9) was not significantly different from the time to peak calcium fluorescence (83.4±7.5 sec, n=9) (Fig. 2F). These results indicate that calcium can permeate the TRPC4 channels and increase calcium concentration near the channels, but not throughout the cytosol. In addition, mTRPC4β-GCaMP6s is a useful tool for testing calcium permeability through TRPC4 channels.
We also investigated whether physiological signaling pathways induce calcium increase through TRPC4 channels (Fig. 3). TRPC4 channels are activated by muscarinic receptor stimulation; thus, we coexpressed the type 2 muscarinic (M2) receptor with mTRPC4β-GCaMP6s. Carbachol (CCh, 100 µM) increased both current and calcium signal with similar time course as EA stimulation (Fig. 3A) showed. The I-V curve showed a typical doubly rectifying shape, consistent with previous reports (Fig. 3B) [2324252627]. The current at -60 mV increased from -10. 6±5.5 pA/pF (n=10) to –476.9±100.4 pA/pF (n=10) (Fig. 3D), while the calcium signal increased from 0.055±0.018 (n=10) to 1.888±0.343 (n=10). The times to peak current (29.1±4.7 sec, n=10) and calcium fluorescence (31.1±4.7 sec, n=10) were both shorter than those in EA stimulation (Fig. 3E). The responses to CCh were of two types. In the first type, the response was rapid and reached its peak around 15 sec (Fig. 3C). In the second type, activation exhibited some delay, and the time to peak was 60 sec (Fig. 3A). The current and calcium signal were well matched, even in this delayed activation phase. These results suggest that Ca2+ influx through the TRPC4 channel either potentiates TRPC4 directly or induces calcium-induced calcium release (CICR), which further activates TRPC4. In situations with the first type of response, CCh effectively induced IP3-mediated Ca2+ release by increasing IP3 level via PIP2 hydrolysis.
We also tested the effect of thapsigargin (TG, 1 µM) on current and calcium signals. TG did not induce a calcium increase in GCaMP6s-tagged TRPC4 (0.090±0.036, n=10) (Fig. 3A). Similarly, TG did not increase the current (–38.6±24.2 pA/pF, n=10). These results suggest that TRPC4 does not act as a SOC, as previously suggested.
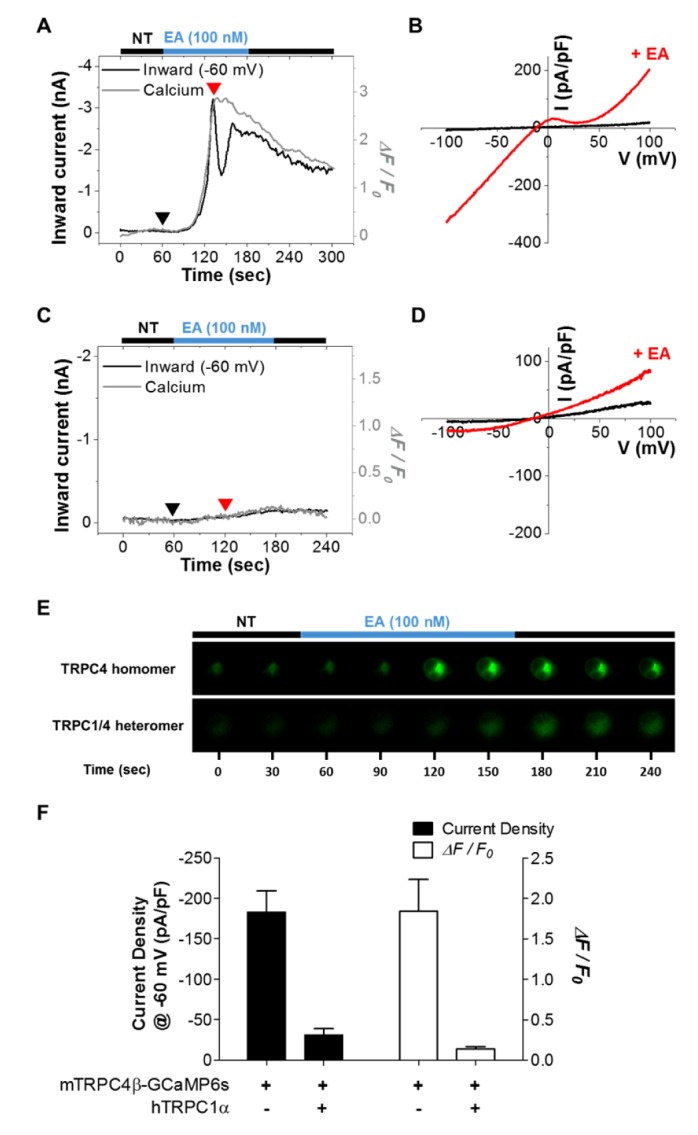
TRPC1 can only mobilize to the plasma membrane when TRPC4 or TRPC5 is coexpressed [2427]. TRPC1 modulates calcium permeability and changes the I-V relationship of the TRPC4 and TRPC5 channels [2428]. To investigate whether TRPC1 affects the detection of calcium near TRPC4 channels, CFP-hTRPC1α was coexpressed with mTRPC4β-GCaMP6s (Fig. 4), and EA was used to activate both TRPC4 homomers and TRPC1/4 heteromers [2021]. EA induced a larger current in TRPC4 homomers (–183.0±26.0 pA/pF, n=10) compared with TRPC1/4 heteromers (–31.2±7.7 pA/pF, n=5) (Fig. 4F). Moreover, the calcium signal increase was greater in TRPC4 homomers (1.84±0.39, n=10) than in TRPC1/4 heteromers (0.141±0.025, n=5). Calcium fluorescence was also visibly decreased in TRPC1/4 heteromers compared to TRPC4 homomers (Fig. 4E). These results indicate that TRPC1 reduces the calcium permeability of TRPC4.
In the present study, we showed that 1) mTRPC4β-GCaMP6s functions as a normal TRPC4 channel, despite the presence of the GCaMP6s at the C-terminus. The I-V curve showed a doubly rectifying shape and reversed at 0 mV. 2) The GCaMP6s attached to mTRPC4β detected calcium near TRPC4 channels, but not cytosolic calcium released from the ER. 3) EA and CCh induce current and calcium both at the similar time courses. 4) Coexpression of TRPC1 with TRPC4 induced a smaller calcium increase than that induced by expression of TRPC4 alone.
The time course of calcium increase matched that of current increase. Ca2+ influx through the mTRPC4β channel contributed to the fluorescence change of GCaMP6s near these channels. There are three types of GCaMP6 with different Kd values, GCaMP6s (144 nM), GCaMP6m (167 nM), and GCaMP6f (375 nM). We used GCaMP6s, which has the lowest Kd value and the highest dynamic range (63). Although GCaMP6m has a similar Kd value, the dynamic range (38) was smaller than that of GCaMP6s [29]. The time course of calcium increase exactly matched that of current increase. In some cells (Fig. 3C and Fig. 4A), the calcium signals were maintained longer than the current signals. This finding might reflect the kinetics of the calcium signal compared with those of the current signal.
The TRPC1 channel regulates the calcium permeability of TRPC4 or TRPC5 when they are coexpressed [28]. We found that heteromerization of TRPC4 with TRPC1 decreased GCaMP6s-mediated detection of calcium increase. TRPC1 has been shown to change the I-V curve of TRPC4 and TRPC5 [242728], but not that of TRPC3/6/7 [28]. TRPC1 reduced the calcium permeability of other TRPC channels. The precise reason why TRPC1 has different effects on the I-V curves of the different TRPC channels remains unclear. Further investigations employing TRPC1 and TRPC3/6/7-GCaMP6s would help clarify whether TRPC1 modulates the calcium permeability of TRPC3/6/7.
Typically, TRPC channel functions have been analyzed by calcium addition experiments after the application of 0 mM Ca2+, especially after Ca2+ store depletion with cyclopiazonic acid (CPA) or TG. In this assay, a store-operated calcium channel serves as the source of calcium. Using these assays, TRPC4 and TRPC5 were suggested to be SOC channels [3031]. STIM and ORAI have been suggested to be the main molecules for SOC channels, especially CRAC channels. In calcium addition experiments, the calcium increase was larger when TRPC4 or TRPC5 channels were expressed compared with control conditions. However, even without TRPC4 expression, the calcium addition protocol induced a calcium increase. The expression of TRPC4 resulted in an additional calcium increase above the basal level. We preferred current measurement to calcium measurement for TRPC4 channels, since calcium measurements always include calcium released from CRAC channels as well as from the expressed channels. Thus, GCaMP6s-tagged TRPC4 or TRPC5 channels could be used as tools for studying structure-function relationships by channel calcium permeability.
TRPC5 has been shown to be activated by intracellular calcium [111232]. Moreover, Ca2+ entry via coexpressed CRAC (STIM1 and Orai1) or L-type voltage-operated Ca2+ channels (VOCC) was sufficient to activate TRPC5 channels, suggesting functional interactions between TRPC5 and other Ca2+-selective channels [32]. We found that TRPC4 was activated by intracellular calcium, similar to TRPC5 (Fig. 3A and 3C). TRPC4 is a candidate nonselective cation channel (NSCC) activated by muscarinic stimulation [3334]. Calcium is well known to facilitate the activation of NSCC, which is in turn activated by muscarinic stimulation [10]. Further experiments are required to determine whether the effect of calcium is direct or mediated by CaM.
The interaction of stromal interaction molecule (STIM) and ORAI, two key components of Ca2+ release-activated Ca2+ (CRAC) channels, with TRPC channels in the formation of diverse SOC channels is still a subject of debate. STIM1 binds to and directly regulates the TRPC1, TRPC4, and TRPC5 channels. TRPC5 was specifically examined as a template ROC to clarify the role of STIM1 in ROC regulation. Knockdown of STIM1 with siRNA specifically suppressed CCh-stimulated, but not La3+-stimulated TRPC5 current [35]. A general model for STIMregulated heteromeric Orai/TRPC in SOC/ICRAC channels has been proposed [36]. Orai1 appears to interact with both the TRPC channel N-terminus and C-terminus; although both channel types can function independently of each other [37], there is growing evidence that Orai1 and TRPCs exist in the same Ca2+ signaling complex and influence the activity of each other [38]. When TRPC1 and TRPC4 formed a heteromeric channel, the N-terminal coiled-coil domain (CCD) and C-terminal region (residues 725~745) of TRPC1 were shown to interact with the N-terminal CCD and C-terminal region (residues 700~728) of TRPC4 [27]. However, when TRPC1 and TRPC5 formed a heteromeric channel, the N-terminal CCD and C-terminal region (residues 673~725) of TRPC1 interacted with the N-terminal CCD and C-terminal region (residues 707~735) of TRPC5. Thus, the N-terminal CCD of TRPC channels is essential for the heteromeric structure of TRPC channels, whereas specific C-terminal regions are required for unique heteromerization between subgroups of TRPC channels [27].
In conclusion, GCaMP6s attached to the C-terminus of TRPC4 can detect slight calcium changes at proximal channels. Thus, TRPC4-GCaMP6s can be a useful tool for testing calcium permeability through TRPC4 channels.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation of Korea, which is funded by the Ministries of Science, ICT (Information & Communication Technology), and Future Planning (MSIP) of the Korea government (2015R1A2A1A05001756 to I.S.). J.Y.K. and J.Y.M. were supported by the BK Plus program of the MSIP.
Notes
References
1. Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989; 2:1313–1323. PMID: 2516726.

2. Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005; 451:1–10. PMID: 16012814.

3. Putney JW. Physiological mechanisms of TRPC activation. Pflugers Arch. 2005; 451:29–34. PMID: 16133266.

4. Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001; 15:1727–1738. PMID: 11481220.
5. Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001; 3:121–127. PMID: 11175743.
6. Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG, Kang DE, Kim HJ, So I. Activation of TRPC4b by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium. 2013; 54:307–319. PMID: 24011658.
7. Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996; 15:6166–6171. PMID: 8947038.

8. Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000; 275:17517–17526. PMID: 10837492.

9. Kim H, Kim J, Jeon JP, Myeong J, Wie J, Hong C, Kim HJ, Jeon JH, So I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin). 2012; 6:333–343. PMID: 22878724.

10. Kim SJ, Koh EM, Kang TM, Kim YC, So I, Isenberg G, Kim KW. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. J Physiol. 1998; 513:749–760. PMID: 9824715.
11. Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005; 280:30788–30796. PMID: 15987684.
12. Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009; 133:525–546. PMID: 19398778.

13. Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DS, Yang DK, So I, Kim KW. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol. 2006; 290:C1031–C1040. PMID: 16306123.

14. Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y. Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol. 2006; 570:219–235. PMID: 16284075.
15. Thakur DP, Tian JB, Jeon J, Xiong J, Huang Y, Flockerzi V, Zhu MX. Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A. 2016; 113:1092–1097. PMID: 26755577.

16. Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012; 3:731. PMID: 22415822.

17. Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001; 31:973–985. PMID: 11580897.
18. Epe B, Steinhäuser KG, Woolley P. Theory of measurement of Förster-type energy transfer in macromolecules. Proc Natl Acad Sci U S A. 1983; 80:2579–2583. PMID: 16593305.
19. Patterson G, Day RN, Piston D. Fluorescent protein spectra. J Cell Sci. 2001; 114:837–838. PMID: 11181166.

20. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791. PMID: 25707820.
21. Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, Yeola S, Lao J, McPate M, Verkuyl JM, Marsh G, Sarber J, Amaral A, Bailey S, Lubicka D, Pham H, Miranda N, Ding J, Tang HM, Ju H, Tranter P, Ji N, Krastel P, Jain RK, Schumacher AM, Loureiro JJ, George E, Berellini G, Ross NT, Bushell SM, Erdemli G, Solomon JM. Englerin a agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One. 2015; 10:e0127498. PMID: 26098886.

22. Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004; 287:C357–C364. PMID: 15044151.
23. Hong C, Kwak M, Myeong J, Ha K, Wie J, Jeon JH, So I. Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. 2015; 467:703–712. PMID: 24859801.

24. Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, Kim SY, Jeon JH, Kim HJ, So I. Isoform- and receptor-specific channel property of canonical transient receptor potential (TRPC)1/4 channels. Pflugers Arch. 2014; 466:491–504. PMID: 23948741.

25. Myeong J, Kwak M, Hong C, Jeon JH, So I. Identification of a membrane-targeting domain of the transient receptor potential canonical (TRPC)4 channel unrelated to its formation of a tetrameric structure. J Biol Chem. 2014; 289:34990–35002. PMID: 25349210.

26. Myeong J, Kwak M, Jeon JP, Hong C, Jeon JH, So I. Close spatio-association of the transient receptor potential canonical 4 (TRPC4) channel with Gαi in TRPC4 activation process. Am J Physiol Cell Physiol. 2015; 308:C879–C889. PMID: 25788576.

27. Myeong J, Ko J, Hong C, Yang D, Lee KP, Jeon JH, So I. The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochem Biophys Res Commun. 2016; 474:476–481. PMID: 27131740.

28. Storch U, Forst AL, Philipp M, Gudermann T, Mederos y Schnitzler M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012; 287:3530–3540. PMID: 22157757.

29. Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013; 499:295–300. PMID: 23868258.

30. Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005; 97:1164–1172. PMID: 16254212.
31. Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, Uhlig S, Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res. 2012; 110:1435–1444. PMID: 22534489.

32. Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalié A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009; 284:34423–34432. PMID: 19815560.
33. Lee KP, Jun JY, Chang IY, Suh SH, So I, Kim KW. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cells. 2005; 20:435–441. PMID: 16404161.
34. Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009; 137:1415–1424. PMID: 19549525.

35. Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007; 9:636–645. PMID: 17486119.

36. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008; 105:2895–2900. PMID: 18287061.

37. DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW Jr. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009; 587:2275–2298. PMID: 19332491.

38. Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010; 584:2022–2027. PMID: 19944100.

Fig. 1
GCaMP6s is suitable for measuring small calcium changes.
(A) Image recordings of GECIs measuring calcium concentration changes (GCaMP6s, R-GECO, YC 6.1 FRET). (B, C) Fluorescence (measured as change in GCaMP6s and R-GECO fluorescence, ΔF, over maximum fluorescence Fmax; ΔF/Fmax) evoked by low calcium (0 mM) and IM (10 µM) in GCaMP6s-transfected (B) or R-GECO-transfected (C) HEK293 cells. The area enclosed by the dashed box includes a magnified form. Each image was captured every 2 sec. (D) FRET efficiency as determined by fluorescence changes in YC 6.1-transfected cells. The conditions are the same as in B~C.
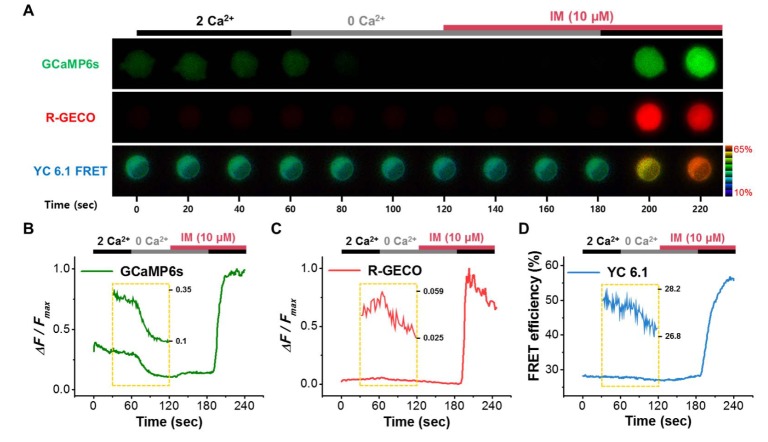
Fig. 2
Simultaneous recordings of the TRPC4 current and channel specific calcium.
(A) Simultaneous recording of calcium influx (grey) and inward current (black) induced by EA (100 nM) in cells transfected with mTRPC4β and GCaMP6s. The maximum calcium response was induced by IM. Calcium is expressed as change in GCaMP6s fluorescence, ΔF, over basal fluorescence F0; ΔF/F0. (B) mTRPC4β-GCaMP6s fusion strategy. GCaMP6s was fused to the C-terminus of mTRPC4β. (C) Full current trace of mTRPC4β-GCaMP6s stimulated by EA. (D) TRPC4-specific calcium influx by mTRPC4β-GCaMP6s. Calcium influx was triggered by EA, thereby stimulating TRPC4 channels. (E) Data from cells transfected with mTRPC4β-GCaMP6s. The conditions are the same as in A. (F) Comparable peak times of current and calcium; blue boxes and bars show means±SEMs.
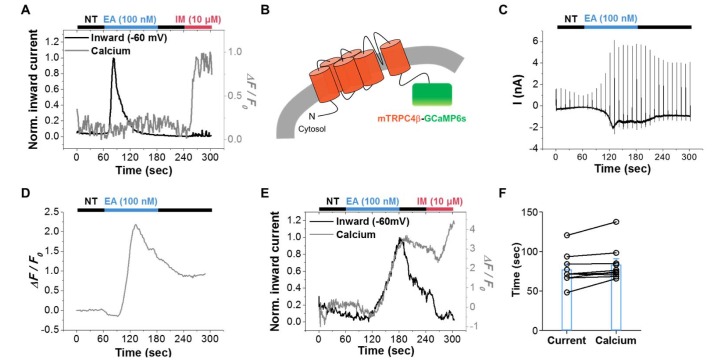
Fig. 3
Calcium increases through TRPC4 mediated by its physiological signaling pathway.
(A) CCh (100 µM) stimulation in mTRPC4β-GCaMP6s and M2 coexpressing cells. TG (1 µM) was added before CCh to block the ability of cells to pump calcium into the ER. (B) I-V curve indicated by the arrow in A. (C) Rapid response to CCh stimulation. The conditions are the same as in A. (D) Comparable peak current densities at –60 mV and peak calcium fluorescence changes induced by TG or CCh for individual cells. (E) Comparable peak times of current and calcium; Blue boxes and bars show means±SEMs.
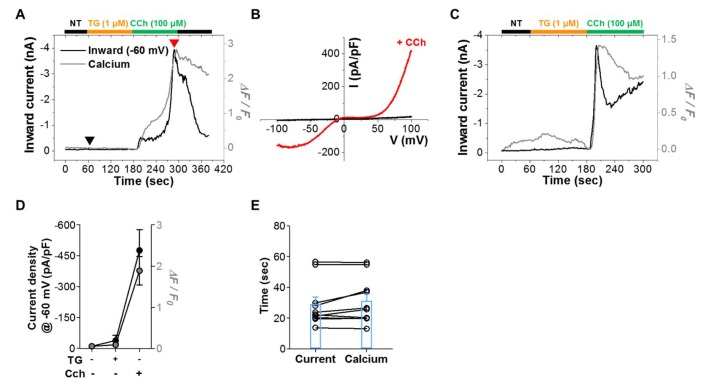
Fig. 4
Reduced current and calcium influx by TRPC1/4 heteromer formation.
(A) Simultaneous recording of calcium influx (grey) and inward current (black) induced by EA (100 nM) in cells transfected with mTRPC4β-GCaMP6s. (B) I-V curve indicated by the arrow in A. (C, D) The conditions are largely the same as in A~B, except that cells were cotransfected with hTRPC1α. (E) Images of GCaMP6s fluorescence in TRPC4 homomer and TRPC1/4 heteromer. (F) Peak current densities at –60 mV and calcium fluorescence changes evoked by EA in TRPC4 homotetramers and TRPC1/C4 heterotetramers.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download