Abstract
The present study was undertaken to investigate the influence of cardamonin on vascular smooth muscle contractility and to determine the mechanism(s) involved. Denuded aortic rings from male rats were used and isometric contractions were recorded and combined with molecular experiments. Cardamonin significantly relaxed fluoride-, phenylephrine-, and phorbol ester-induced vascular contractions, suggesting that it has an anti-hypertensive effect on agonist-induced vascular contraction regardless of endothelial nitric oxide synthesis. Furthermore, cardamonin significantly inhibited the fluoride-induced increase in pMYPT1 level and phenylephrine-induced increase in pERK1/2 level, suggesting inhibition of Rho-kinase and MEK activity and subsequent phosphorylation of MYPT1 and ERK1/2. This study provides evidence that the relaxing effect of cardamonin on agonist-induced vascular contraction regardless of endothelial function involves inhibition of Rho-kinase and MEK activity.
Cardamonin ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one, Fig. 1) is the main flavonoid derived from the seed of Alpinia katsumadai (Zingiberaceae), which is a commonly used traditional medicinal plant in China and Korea. Previous studies have demonstrated that cardamonin inhibits the proliferation of various cancer cells [1] and inhibits the proliferation of vascular smooth muscle cells and the production of pro-inflammatory mediators [2]. We investigated the mechanisms underlying the relaxing effect of cardamonin on vascular smooth muscle contractility with the ultimate goal of developing a better antihypertensive agent. Denuded aortic rings from male Sprague-Dawley rats were used and isometric contractions were recorded using a computerized data acquisition system. These data were combined with molecular experiments.
It is generally accepted that initiation of smooth muscle contractility is predominantly controlled by a Ca2+-dependent increase in the phosphorylation of the 20 kDa myosin light chain (MLC20) [3]. However, the degree of MLC20 phosphorylation or contraction does not always parallel the intracellular Ca2+ concentration. The extent of MLC20 phosphorylation or force of contraction induced by agonist stimulation is usually higher than that caused by an increase in the Ca2+ concentration, which is referred to as Ca2+ sensitization [3]. Subsequent studies suggested that inhibition of MLC phosphatase by Rho-kinase [4567] or thin filament regulation, including the activation of protein kinase C (PKC), mitogen-activated protein kinase kinases (MEK), and extracellular signal regulated kinase (ERK) 1/2, as well as phosphorylation of the actin binding protein caldesmon [8] may be major components of the pathway that facilitates Ca2+ sensitization.
Fluoride or phorbol ester has been shown to induce contraction of various smooth muscles, which may be due to enhanced Ca2+ sensitivity. In particular, fluoride induces contractions in blood vessel preparations and is known to be a potent stimulator of Gs, Gi, Gq, and transducin [91011]. It is possible that the contractions induced by fluoride involve the RhoA/Rho-kinase pathway [12]. However, it is not known whether this pathway is inhibited during cardamonin-induced vascular smooth muscle relaxation in aortic rings precontracted with Rho-kinase activator fluoride or phorbol ester. Therefore, the aim of the present study was to investigate the possible roles of Rho-kinase and MEK inhibition on Ca2+ desensitization during cardamonin-induced relaxation of isolated rat aortas by using RhoA/Rho-kinase activators such as fluoride and phenylephrine (full activators) and a phorbol ester (partial activator).
Male Sprague-Dawley rats weighing 200~250 g were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and then subjected to cervical dislocation, in accord with the procedures approved by the Institutional Animal Care and Use Committee at our institutions. Thoracic aortas were removed quickly and immersed in oxygenated (95% O2/5% CO2) physiological saline solution composed of (mM): 115.0 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25.0 NaHCO3, 1.2 KH2PO4, and 10.0 dextrose (pH 7.4). The aortas were then freed of all adherent connective tissue, and aortic endothelia were removed by gentle abrasion using a cell scraper.
Care was taken to avoid rubbing the endothelial surface of the vessels with intact endothelium. Two stainless steel triangles were inserted through each vessel ring and each aortic ring was then suspended in a water-jacketed organ bath (10 ml) maintained at 37℃ and aerated with a mixture of 95% O2 and 5% CO2. One triangle was anchored to a stationary support, and the other was connected to an isometric force transducer (Grass FT03C, Quincy, MA, USA). The rings were stretched passively by applying an optimal resting tension of 2.0 g, which was maintained throughout the experiment. Each ring was equilibrated in the organ bath solution for 60 min before contractile responses to 50 mM KCl or 1 µM phenylephrine were measured. Isometric contractions were recorded using a computerized data acquisition system (PowerLab/8SP, AD Instruments, Castle Hill, NSW, Australia).
The direct effect of cardamonin was determined by addition of it after KCl- (50 mM), phenylephrine- (1 µM), phorbol ester- (1 µM), or fluoride- (6 mM) induced contractions had plateaued in normal Krebs' solution.
Muscle strips were quick-frozen by immersion in a dry ice/acetone slurry containing 10% trichloroacetic acid (TCA) and 10 mM dithiothreitol (DTT). Muscles were stored at –80℃ until use. Tissues were brought up to room temperature in a dry ice/acetone/TCA/DTT mixture and then homogenized in a buffer containing 20 mM MOPS, 4% SDS, 10% glycerol, 10 mM DTT, 20 mM β-glycerophosphate, 5.5 µM leupeptin, 5.5 µM pepstatin, 20 kIU aprotinin, 2 mM Na3VO4, 1 mM NaF, 100 µM ZnCl2, 20 µM 4-(2-aminoethyl) benzenesulphonyl fluoride (AEBSF), and 5 mM EGTA. Protein-matched samples (modified Lowry protein assay, DC Protein Assay Kit, Bio-Rad) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Protogel, National Diagnostics), transferred to polyvinylidene fluoride (PVDF) membranes, and subjected to immunostaining and densitometry using primary and secondary antibodies. Lane loading variations were corrected by normalization to β-actin. Sets of samples produced during individual experiments were run on the same gel, and densitometry was performed on the same image.
Drugs and chemicals were obtained from the following sources. Sodium fluoride, KCl, acetylcholine, cardamonin, phenylephrine, and phorbol 12,13-dibutyrate were purchased from Sigma (St. Louis, MO, USA). DTT, TCA, and acetone were obtained from Fisher Scientific (Hampton, NH, USA). Enhanced chemiluminescence (ECL) kits were from Pierce (Rockford, IL, USA). Antibodies against phospho-myosin phosphatase-targeting subunit protein 1 (phospho-MYPT1) at Thr855 (1:5,000), MYPT1, ERK, or phosphoERK at Thr202/Tyr204 were purchased from Cell Signaling Technology (Danvers, MA, USA) or Upstate Biotechnology (Lake Placid, NY, USA) to determine levels of RhoA/Rho-kinase activity [1314] or MEK activity. Anti-mouse IgM (goat) and anti-rabbit IgG (goat) conjugated with horseradish peroxidase were used as secondary antibodies (1:2,000 and 1:2,000, respectively, Upstate). A specific MLC20 antibody (1:1500, Sigma) and anti-mouse IgG (Goat) conjugated with horseradish peroxidase (1:2000, Upstate) were used to determine the level of LC20 phosphorylation. Cardamonin was prepared in dimethyl sulfoxide (DMSO) as a 100 mM stock solution and frozen at –20℃ for later use. DMSO alone had no observable effect at the concentrations used (data not shown).
Data are expressed as means±standard errors of the mean (SEMs). Student's unpaired t test or two-way ANOVA was used to determine the statistical significance of differences in means between two groups using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). p values<0.05 were regarded as statistically significant.
Endothelium was removed by gentle abrasion with a cell scraper to identify the direct effect of cardamonin on vascular smooth muscle. The absence of endothelium was confirmed by a lack of relaxation after treating precontracted ring segments with acetylcholine (1 µM). Cardamonin had no significant effect on basal tension (data not shown), but significantly inhibited the contraction induced by the full activator fluoride at a low concentration, regardless of endothelial nitric oxide synthesis (Fig. 2A) or with intact muscles (Fig. 2B). This suggested that the relaxation effect of cardamonin might involve inhibition of Rhokinase activity in addition to endothelial nitric oxide synthesis and the subsequent activation of guanylyl cyclase. Cardamonin at the same concentration inhibited phenylephrine-induced contraction (Fig. 3), suggesting that phenylephrine acts similarly to a full activator when Rho-kinase activation is the main underlying pathway.
Phorbol esters have been shown to be MEK activators and partial RhoA/Rho-kinase activators [15]. Phorbol 12,13-dibutyrate-induced contraction was significantly inhibited by cardamonin at a low concentration, regardless of endothelial nitric oxide synthesis (Fig. 4), which suggested that thin or actin filament regulation by MEK/ERK was significantly inhibited.
To confirm the role of cardamonin on thick filament regulation of smooth muscle contractility, we measured levels of myosin phosphatase targeting subunit 1 (MYPT1) and phospho-MYPT1 in muscles quick-frozen after a 60-min exposure to cardamonin for equilibration. Each relaxing ring was precontracted with 6 mM fluoride. This work was done using quick-frozen cardamonin (0.1 mM)-treated rat aortas in the absence of endothelium and levels were compared to those of vehicle-treated rat aortas (Fig. 5). A significant decrease in fluoride-induced MYPT1 phosphorylation at Thr855 in response to cardamonin treatment was observed (Fig. 5). Thus, thick or myosin filament regulation, including myosin phosphatase activation via RhoA/Rho-kinase inactivation, might be involved in the reduced contractility of cardamonin-treated rat aorta. The intensity of relaxation appeared to be directly proportional to the level of inhibition of MYPT1 phosphorylation by cardamonin.
To further examine the effects of cardamonin on thin filament regulation of smooth muscle contractility, we measured levels of ERK1/2 and phospho-ERK1/2 in quick-frozen muscles after a 60-minute exposure to cardamonin for equilibration. Each relaxing ring was precontracted with 1 µM phenylephrine. As compared with vehicle-treated rat aortas, a significant decrease in ERK 1/2 phosphorylation at Thr202/Tyr204 was observed in cardamonin (0.1 mM)-treated rat aortas in the absence of endothelium (Fig. 6); full vasorelaxation (Fig. 4) and thin filament regulation were observed. These findings indicate that thin or actin filament regulation by ERK1/2 phosphorylation via MEK activation does also play an important role in cardamonin-induced relaxation.
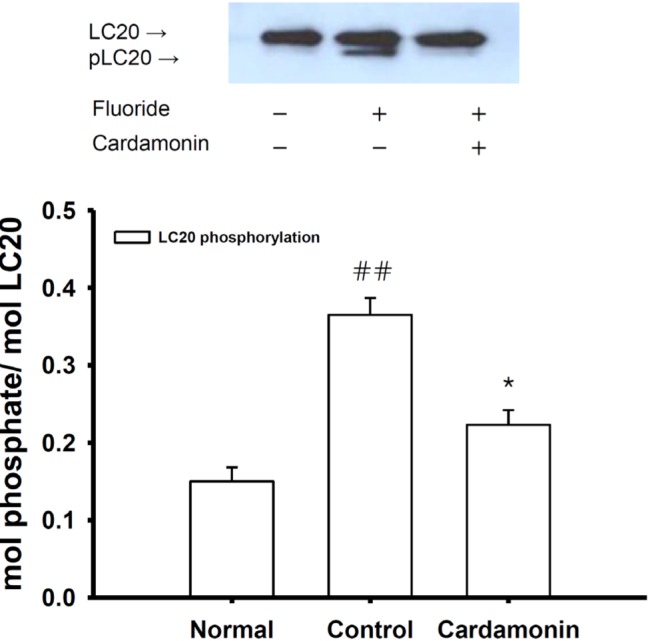
To investigate if cardamonin affected fluoride-induced MLC20 phosphorylation, aortic rings were treated with cardamonin after the addition of fluoride. Each relaxing ring was precontracted with 6 mM sodium fluoride. This work was done using quick-frozen cardamonin (0.1 mM)-treated rat aortas in the absence of endothelium, and levels were compared to those of vehicle-treated rat aortas (Fig. 7). A significant decrease in fluoride-induced LC20 phosphorylation was found in response to cardamonin treatment (Fig. 7). Thus, the reduced contractility of cardamonin-treated rat aorta may involve thick or myosin filament regulation including calcium immobilization and MLCK inactivation.
In the present study, we demonstrated that cardamonin can modulate vascular contractility in an agonist-dependent manner. Interestingly, the mechanisms involved seem not only to be endothelium-dependent, but also to involve inhibition of MEK and Rho-kinase activity. Cardamonin has previously been recognized for its anti-inflammatory and antinociceptive properties. Therefore, we investigated whether the inhibition of RhoA/Rho-kinase or MEK activity contributed to cardamonin-induced vascular relaxation in rat aortas denuded and precontracted by a RhoA/Rho-kinase activator (fluoride) or by MEK activators (phenylephrine or phorbol ester).
The mechanism by which phorbol ester activates MEK/ERK has been established [1617]. In contrast, previous studies that examined the mechanisms underlying arterial contractions induced by fluoride or phenylephrineB have reported various findings with regard to the contraction triggered by Rho-kinase activation [1318]. Our findings are consistent with the notion that cardamonin can decrease phorbol ester-, phenylephrine-, and fluoride-induced contraction by inhibiting MEK or Rho-kinase activity.
The mechanisms by which MEK activation causes vascular contraction is an area of intense study, and several possibilities exist. Phosphorylation of caldesmon by MEK/ERK appears to regulate smooth muscle contractility [17]. In this process, MEK/ERK is activated by PKC, which in turn can be stimulated by phorbol esters or GPCR receptor agonists.
We demonstrated that cardamonin ameliorated maximal or submaximal contractions induced by vasoconstrictors (fluoride or phorbol ester) independent of the endothelium (Figs. 2, and 4), and that this ameliorative mechanism involved the MEK/ERK and RhoA/Rho-kinase pathway. Previously, most vasodilation was believed to be caused by endothelial nitric oxide synthesis and the subsequent activation of guanylyl cyclase [1920]. In the present study, cardamonin at a low concentration significantly inhibited phorbol ester- or fluorideB-induced contractions, regardless of endothelial function (Figs. 2, and 4). Furthermore, cardamonin decreased the phenylephrine-induced phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 (Fig. 6) and significantly decreased the fluoride-induced phosphorylation of MYPT1 at Thr855 (Fig. 5) with full relaxation (Fig. 2), suggesting that inhibition of Rho-kinase or MEK activity is a major mechanism underlying the relaxing effect of cardamonin. MLC20 is known to be phosphorylated by both MLCK and Rho-kinase [21]. Activation of Rho-kinase by phenylephrine or fluoride inhibits the activity of myosin light chain phosphatase through phosphorylation of MYPT1, leading to an increase in MLC20 phosphorylation as well as contractions [613]. Both phenylephrine and fluoride phosphorylated MLC20, and this was inhibited by cardamonin (Fig. 7).
In summary, cardamonin at a low concentration significantly attenuated contractions induced by MEK activators (phenylephrine or a phorbol ester) regardless of endothelial function. Furthermore, Rho-kinase activator (fluoride)-induced contractions were significantly inhibited by this low concentration of cardamonin. Thus, the mechanism underlying the relaxation induced by a low concentration of cardamonin of phorbol ester-, phenylephrine-, or fluoride-induced contractions involves inhibition of MEK activity and Rho-kinase activity. Inhibition of Rho-kinase activity and subsequent MYPT1 phosphorylation induced by cardamonin during fluoride-induced contraction suggested that Rho-kinase inactivation is required for relaxation. In conclusion, in addition to endothelial nitric oxide synthesis in intact muscle, both MEK and Rho-kinase inhibition contribute synergistically to cardamonin-induced vasorelaxation in denuded muscle.
References
1. Gonçalves LM, Valente IM, Rodrigues JA. An overview on cardamonin. J Med Food. 2014; 17:633–640. PMID: 24433078.

2. Chow YL, Lee KH, Vidyadaran S, Lajis NH, Akhtar MN, Israf DA, Syahida A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int Immunopharmacol. 2012; 12:657–665. PMID: 22306767.

3. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372:231–236. PMID: 7969467.

4. Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998; 164:437–448. PMID: 9887967.

5. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997; 389:990–994. PMID: 9353125.

6. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003; 93:548–556. PMID: 12919947.
7. Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991; 88:9307–9310. PMID: 1656467.

8. Wier WG, Morgan KG. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003; 150:91–139. PMID: 12884052.
9. Kanaho Y, Moss J, Vaughan M. Mechanism of inhibition of transducin GTPase activity by fluoride and aluminum. J Biol Chem. 1985; 260:11493–11497. PMID: 2995338.

10. Blackmore PF, Exton JH. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986; 261:11056–11063. PMID: 2426266.

11. Cockcroft S, Taylor JA. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987; 241:409–414. PMID: 3036062.
12. Jeon SB, Jin F, Kim JI, Kim SH, Suk K, Chae SC, Jun JE, Park WH, Kim IK. A role for Rho kinase in vascular contraction evoked by sodium fluoride. Biochem Biophys Res Commun. 2006; 343:27–33. PMID: 16527249.

13. Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rhoassociated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005; 389:763–774. PMID: 15823093.
14. Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004; 279:34496–34504. PMID: 15194681.

15. Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol. 2009; 297:H2242–H2252. PMID: 19749163.

16. Gu Z, Kordowska J, Williams GL, Wang CL, Hai CM. Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp Cell Res. 2007; 313:849–866. PMID: 17239373.

17. Kordowska J, Huang R, Wang CL. Phosphorylation of caldesmon during smooth muscle contraction and cell migration or proliferation. J Biomed Sci. 2006; 13:159–172. PMID: 16453176.

18. Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. 2006; 453:223–232. PMID: 16953424.

19. Ajay M, Gilani AU, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003; 74:603–612. PMID: 14623031.

20. Taubert D, Berkels R, Klaus W, Roesen R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: essential structural features. J Cardiovasc Pharmacol. 2002; 40:701–713. PMID: 12409979.

21. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83:1325–1358. PMID: 14506307.
Fig. 1
The chemical structure of cardamonin ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one).
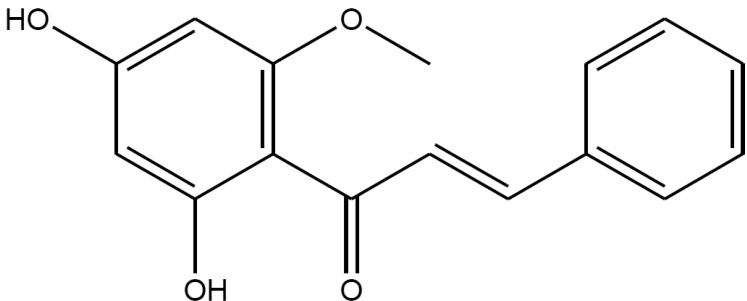
Fig. 2
Effect of cardamonin on fluoride-induced vascular contraction in denuded (A) or intact (B) muscles.
Each ring was equilibrated in organ bath solution for 30~60 min before relaxation responses to cardamonin were measured. Data are expressed as the means of 3~5 experiments with vertical lines representing SEMs. *p<0.05, **p<0.01, presence versus absence of cardamonin.
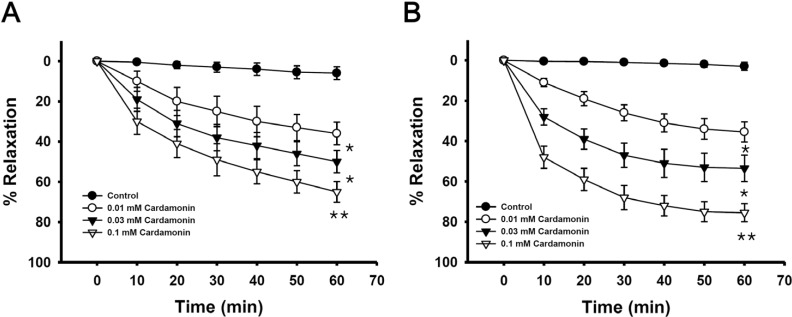
Fig. 3
Effect of cardamonin on phenylephrine-induced vascular contraction.
Each ring was equilibrated in organ bath solution for 30~60 min before relaxation responses to cardamonin were measured. Data are expressed as the means of 3~5 experiments with vertical lines representing SEMs. *p<0.05, **p<0.01, presence versus absence of cardamonin.
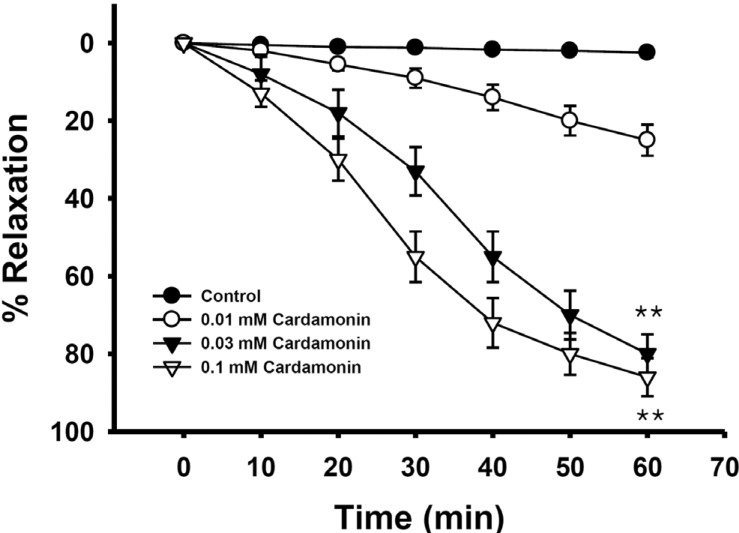
Fig. 4
Effect of cardamonin on phorbol ester-induced vascular contraction.
Each ring was equilibrated in organ bath solution for 30~60 min before relaxation responses to cardamonin were measured. Data are expressed as the means of 3~5 experiments with vertical lines representing SEMs. *p<0.05, **p<0.01, presence versus absence of cardamonin.
Fig. 5
Effect of cardamonin on fluoride-induced increases in phospho-MYP T1 levels.
Phospho-MYPT1 protein levels were significantly decreased in rapidly-frozen cardamonin-treated rat aorta in the absence of endothelium compared to vehicle-treated rat aorta precontracted with fluoride. The upper panel shows a typical blot and the lower panel shows average densitometry results for relative levels of phospho-MYPT1. Data are expressed as the means of 3~5 experiments with vertical lines representing SEMs. *p<0.05, ##p<0.01, versus control or normal group respectively. Carda: 0.1 mM cardamonin; Fluoride: 6 mM sodium fluoride.
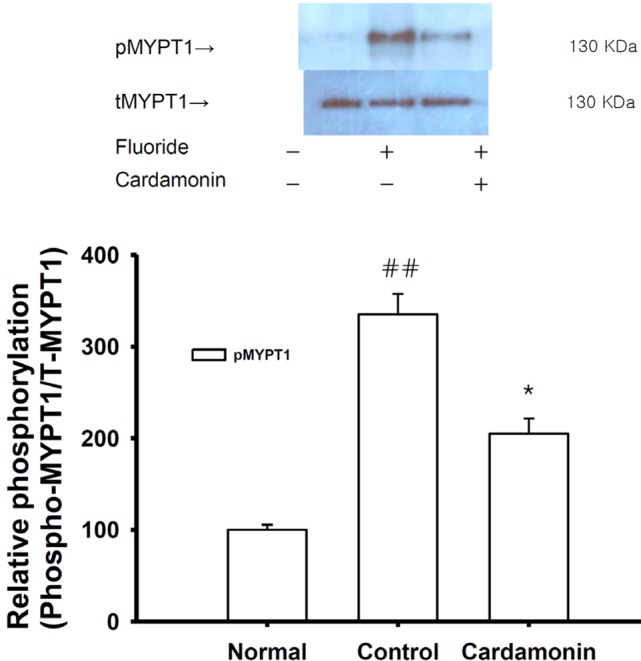
Fig. 6
Effect of cardamonin on the phenylephrine-induced increase in phospho-ERK1/2 level.
Phospho-ERK1/2 protein level was decreased in rapidly-frozen cardamonin-treated rat aortas in the absence of endothelium than vehicle-treated rat aortas precontracted with a phorbol ester. The upper panel shows a typical blot and the lower panel shows average densitometry results for relative levels of phospho-ERK1/2. Data are expressed as the means of 3-5 experiments with vertical lines representing SEMs. ##p<0.01, versus normal group respectively. Cardamonin: 0.1 mM cardamonin; Phenylephrine: 1 µM phenylephrine.
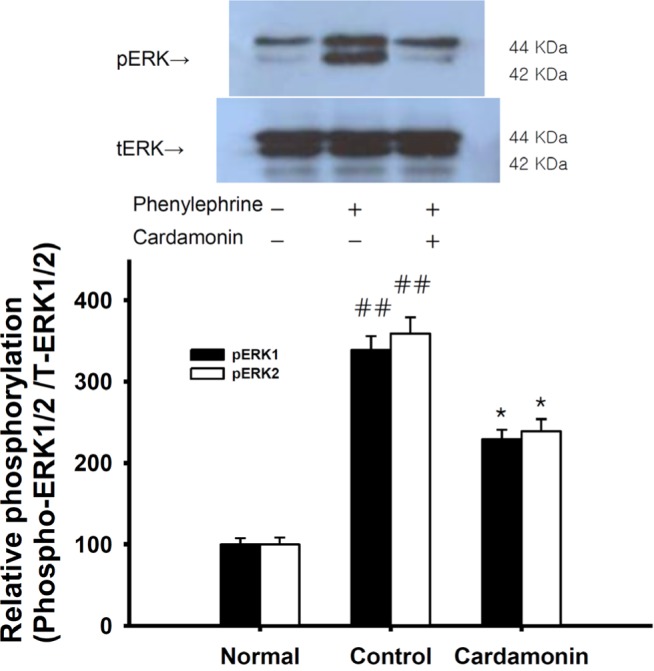
Fig. 7
Effect of cardamonin on the fluoride-induced increase in phospho-MLC20 level.
Phospho-MLC20 levels expressed as a percentage of total MLC20 were significantly lower in rapidly-frozen cardamonin-treated rat aorta in the absence of endothelium than vehicle-treated rat aorta precontracted with fluoride (6 mM). Data are expressed as the means of 3~5 experiments with vertical lines representing SEMs. *p<0.05, ##p<0.01, versus control or normal group respectively. Cardamonin: 0.1 mM cardamonin; Fluoride: 6 mM sodium fluoride.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download