Abstract
Metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD), a type of synaptic plasticity, is characterized by a reduction in the synaptic response, mainly at the excitatory synapses of the neurons. The hippocampus and the cerebellum have been the most extensively studied regions in mGluR-dependent LTD, and Group 1 mGluR has been reported to be mainly involved in this synaptic LTD at excitatory synapses. However, mGluR-dependent LTD in other brain regions may be involved in the specific behaviors or diseases. In this paper, we focus on five cortical regions and review the literature that implicates their contribution to the pathogenesis of several behaviors and specific conditions associated with mGluR-dependent LTD.
Synaptic plasticity, which is the strengthening or the weakening of the synaptic response to stimulus, has attracted considerable attention from neuroscientists investigating various aspects of brain function. Long-term potentiation (LTP) and long-term depression (LTD) are the two major forms of synaptic plasticity observed in electrophysiology and other investigative studies examining at the synapses of the neurons [1]. The hippocampus, especially the region of Schaffer collateral–CA1 projection fibers, is the most well-studied region in the central nervous system for synaptic plasticity, and the mechanisms of LTP and LTD have already been well established. Synaptic plasticity in the hippocampus is one of the most widely recognized cellular and synaptic model of learning and memory in the neuroscience field and continues to be adapted in different behavioral or disease models for various brain regions.
LTD is the activity-dependent reduction in the efficacy and strength of synaptic transmission lasting for a few hours or longer. There are two major forms of LTD in the CNS: the N-methyl-D-aspartate receptor (NMDAR)-dependent LTD [23] and NMDAR-independent or mGluR-dependent LTD [456]. These can be induced by different stimulating protocols in different regions and have been best investigated in the hippocampus and cerebellum.
mGluR-dependent LTD was first observed at the synapses of the cerebellar parallel fiber—Purkinje cells [789]. mGluR-dependent LTD involves the activation of mGluRs, which can be classified into three groups: Group I comprises mGluR1 and mGluR5, which are mainly present in the postsynaptic site [10] and have been mainly investigated in the context of LTD; Group II includes mGluR2 and mGluR 3; and Group III consists of mGluR4, mGluR6, mGluR7, and mGluR8. Groups II and III mGluRs are mainly expressed in the presynaptic site. Previous studies have shown that mGluR-dependent LTD can be induced after activation of mGluR1, mGluR5, mGluR2, mGluR3, and mGluR7 [6]. In this review, we will focus on mGluR-dependent LTD, especially in the cortical regions.
Two major methods are used to induce mGluR-dependent LTD. First is the application of chemicals that stimulate mGluRs. Acute administration of Group I mGluR agonist 3,5-dihydroxyphenylglycine (DHPG) is the most common method to induce mGluR-dependent LTD throughout the brain [1112]. Group II mGluR needs to be activated in some regions and Group II mGluR agonist 2S, 2'R, 3'R)-2-(2', 3'-dicarboxycyclopropyl) glycine (DCG-IV) is one of the most well-known drugs capable of inducing LTD in the prefrontal cortex [1314].
The second method of inducing mGluR-dependent LTD is low-frequency stimulation (LFS). 900 single pulses in 1 Hz usually induces NMDAR-dependent LTD, and 900 pairs of 50-ms-apart pulses in 1 Hz are known to induce mGluR-dependent LTD [151617]. In the CA1 region of the hippocampus, paired-pulse LFS (PP-LFS) activates Group I mGluRs and M1 muscarinic acetylecholine receptors [151819]. However, in some cortical regions, such as the anterior cingulate cortex (ACC) and insular cortex (IC), 900 single pulses in 1 Hz induce LTD that are fully blocked by (S)-α-methyl-4-carboxyphenylglycine (MCPG), which is an antagonist to Groups I and II mGluRs [202122]. In addition, there are also other unique protocols such as the application of mGluR agonists and electrical stimulation with tetanus [2324] or using weaker LFS and maintaining the postsynaptic potential at –40 mV which is called the pairing protocol [2526].
LTD dependent on Group I mGluR is the most studied form of mGluR-dependant LTD throughout the brain and has been extensively investigated by neuroscientists. Stimulation of Group I mGluR activates phospholipase C (PLC), inositol triphosphate (IP3) pathway to release Ca2+ from intracellular stores and protein kinase C (PKC) [272829]. PKCα phosphorylates ser880 of GluA2 to trigger endocytosis of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and reduce the level of surface expression [29303132]. Several other protein elements such as p38 mitogen-activated protein kinase (p38 MAPK) [3334
35], extracellular signal-regulated kinases (ERKs) [36], Arc [3738], striatal-enriched protein phosphatase (STEP) [29394041], phosphoinositide 3-kinase (PI3K) [42], protein tyrosine phosphatases (PTP) [34] are also known to be involved in Group I mGluR-dependent LTDs. Several detailed reviews of the mechanism of mGluR-dependent LTD have been published previously [6124344].
mGluR-dependent LTD in the hippocampus and the cerebellum have been extensively reviewed previously [6124344]. Here, we present an overview of the less-reviewed cortical regions.
The anterior cingulated cortex (ACC) is known to participate in a wide variety of functions in the brain such as cognition, error detection, decision making, memory, emotion, and pain [45464748
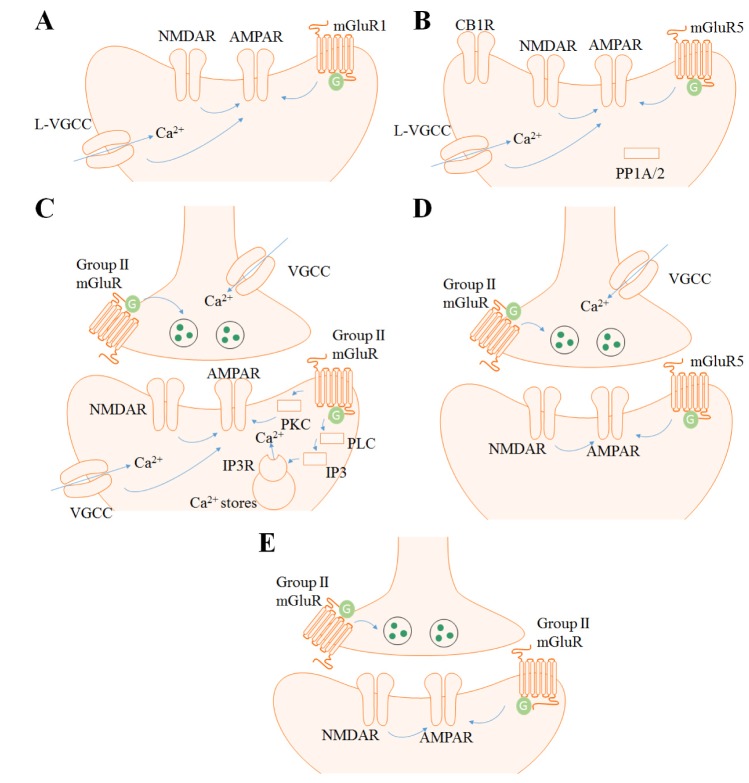
49]. Numerous human brain imaging studies have investigated the role of ACC in these functions, however, studies on synaptic plasticity in animals are mainly restricted to the pain field. The ACC consists of five layers (I, II, III, V, and VI) and the excitatory pyramidal neurons are mainly positioned in the layer II/III and V. Layer II/III pyramidal neurons mostly receive sensory inputs from the medial thalamus and project to layer V/VI neurons. Layer V pyramidal neurons receive inputs from layer II/III and the thalamus and send projections to other cortical regions. Numerous local interneurons such as parvalbumin (PV) and somatostatin (SOM) positive interneurons are localized in layer I and II-VI [5051]. Field recordings in rat and mice have shown that LTD is induced in the ACC with 1-Hz single-pulse LFS for 15 min and had similar results with several pharmacological studies [2022]. Field recordings in rat were performed in the layer II, and the recordings in the mouse was done throughout the ACC layers. Treatment with MGPC (500 µM), an antagonist to mGluR of Groups I and II, inhibited this LTD in both rat and mice, but the NMDAR antagonist APV (50 µM) was not able to completely block LTD. Unlike the case with the hippocampus, single-pulse LFS in the neural populations of the ACC induced strong synaptic depression, and the NMDAR function was less important. Moreover, the mGluR5 antagonist MPEP (10 µM) had no effect on the ACC neurons, whereas the mGluR1 antagonist LY 367385 (100 µM) fully blocked this LFS-induced LTD. L-type voltage gated calcium channel (L-VGCC) blocker also inhibited the LTD in the ACC induced by single-pulse LFS. These results imply that single-pulse LFS induced by LTD in the ACC depends on mGluR1 and L-VGCC (Fig. 1A). LTD was also induced by combined treatment with DHPG (100 µM) and MPEP (10 µM) for 20 min. When a digit of hind paw or 2.5 cm of the tail was amputated, LTD induced by LFS as well as chemical LTD induced by DHPG+MPEP were impaired. Chronic peripheral injury was involved in the disruption of the mGluR-dependent synaptic plasticity in the ACC, and this could be one of the functional characteristics in a phantom pain models brain. By priming the ACC slice of the amputated animal with low-dose DHPG (20 µM) and MPEP (10 µM) before the application of LFS, the impairment in the LTD was rescued [20]. This priming effect was PKC dependent. This type of metaplastic application could be a possible direction for the further development of treatment strategies in pain-related situations.
IC is also a region known for its diverse functions, including perception, motor control, self-awareness, and cognitive functioning. Several behavioral studies such as those pertaining to conditioned taste aversion [525354] and pain [555657] have been conducted in the past. However, only few IC studies address synaptic plasticity [585960]. IC is divided into granular (GI), dysgranular (DI) and agranular (AI) cortices. All are composed of five layers as the ACC except the GI which has six layers including layer IV [61]. Pyramidal neurons are found mainly in the layer III and V [62] and there are also GABAergic interneurons in the layer V [63]. The afferent and efferent projections of the IC are well studied throughout the layers in several animal models although the cell type composition within layers still needs to be investigated further.
Two studies [2164] was performed in a manner similar to that of Kang et al. 2012. Recordings were done throughout all the layers in the IC and was plotted as superficial (layer I~II/III) and deep layer (layer V~VI). LTD in the IC was induced by 1-Hz single-pulse LFS for 15 min, and this LTD was blocked by APV (50 µM), nimodipine (10 µM), a selective cannabinoid 1 receptor (CB1R) antagonist AM251 (5 µM) and a selective protein phosphatase 1/2A (PP1/2A) inhibitor okadaic acid (1 µM). Interestingly, it was also fully blocked by MPEP (10 µM) but not CPCCOEt (100 µM), a selective mGluR1 antagonist. Therefore, LFS induced LTD in the IC is dependent on NMDAR, L-VGCC, CB1R, PP1/2A and mGluR5, when the same stimulating protocol induced mGluR1-dependent LTD in the ACC (Fig. 1B). DHPG (100 µM, 20 min) was also able to induce chemical LTD in the IC. This LTD was blocked when nimodipine (10 µM) was administered 20 min before DHPG infusion, thereby implying the importance of L-VGCC in DHPG-LTD. There was no layer difference in the experiments.
When the tail was amputated, LFS induced LTD was blocked in IC as ACC, but chemical LTD induced by DHPG remained unchanged [64]. Therefore, tail amputation might have more effect in the NMDAR than mGluR5 in IC. However, the impaired LFS-induced LTD could be improved by mGluR-dependent metaplasticity. Application of DHPG (20 µM) had no effect at the baseline, but subsequent LFS was able to induce LTD in the IC slice of the amputated mice. This priming effect was PKC dependent.
The prefrontal cortex (PFC) is a region known for working memory [656667], attention [686970] and executive function [717273]. Numerous studies have investigated this region on several topics and is a popular research target because of its variety of functions. There are many more studies on mGluR-dependent LTD in PFC than in the ACC or IC; and few will be reviewed here. PFC consists of five layers as the ACC. Layer I contains neuropil, axons and GABAergic interneurons. Layers II/III and V/VI are composed of pyramidal neurons and various interneuron types [74].
Most of the mGluR LTD in the PFC is dependent on Group II mGluR. The Group I mGluR agonist DHPG (100 µM, 10~15 min) was not able to induce LTD in the prefrontal slice, but acute bath application of the potent Group II mGluR agonist DCG IV (50~100 nM) showed depression of the synaptic response for more than 40 min in layer I-II to layer V pyramidal neuron glutamatergic synapses of rat PFC [75]. This mGluR-dependent LTD was blocked when the postsynaptic cell was injected with Ca2+ chelator BAPTA (100 mM). It was also blocked when APV (100 µM) was applied together with DCG IV [14]. Moreover, the phospholipase C inhibitor U-73122 (4 µM, bath application), IP3 receptor blocker heparin (4 mg/ml in recording electrode targeting postsynaptic neuron), PKC inhibitor RO318220 (0.2 µM, bath application), all impaired the LTD. These results suggest that intracellular calcium, NMDAR-mediated responses, phospholipase C, IP3 receptor, and PKC together contribute to LTD induced by DCG IV (Fig. 1C).
LTD induced by DCG IV (0.2 µM, 10 min) in layer V pyramidal neurons was impaired when rats were treated with cocaine repeatedly for more than 5 days [13]. This impairment of LTD was improved when selective D1-like receptor antagonist SCH23390 (0.5 mg/kg) was coadministered with cocaine. Moreover, bilateral intra-mPFC infusion of PKC inhibitor bisindolylmaleimide I (0.4 nmol/side) or adenosine A3 receptor antagonist MRS1220 (0.5 nmol/side) also improved the impaired LTD. These results indicate that repetitive cocaine exposure inhibits Group II mGluR function via D1-like receptor, PKC, and A3 receptor.
A study on the importance of mGuR3 in LTD at PFC layer V pyramidal neurons and fear extinction has also been published [76]. Bath application of Group II agonist LY379268 (0.1 µM, 10 min) was able to induce LTD in the mice PFC slice. This LTD was blocked when mGluR3-negative allosteric modulator VU0469942 (10 µM) or VU0477950 (10 µM) was applied. When VU0477950 (3~100 mg/kg) was intraperitonially injected, fear extinction learning was impaired.
The perirhinal cortex is the region situated within the medial temporal lobe and is involved in visual perception, memory, and several types of learning [7778]. Ablation studies in perirhinal cortex of rats and primates show impairment in recognition memory tasks; however, such studies on the hippocampal lesions showed less effect [79808182]. Perirhinal cortex is composed of agranular cortex with five layers and dysgranular cortex with six layers, but the cell types within this region have not been well studied yet [83].
The perirhinal cortex showed mGluR-dependent LTD when DHPG (50 µM, 20 min) or DGC IV (1 µM, 20 min) was applied; however, Group III mGluR agonist L-AP4 (0.1~1 mM) showed only acute depression in the superficial layer (layer I and II/III) during drug application [84]. LFS (200 stimuli, 1Hz) with depolarization of postsynaptic neuron to –40 mV also induced LTD in the layer II/III neurons of perirhnial cortex [25]. This LTD was blocked by APV (50 µM), MCPG (500 µM), Group I mGluR antagonist AIDA (500 µM). The Group II mGluR antagonist EGLU (200 µM) only blocked the LTD when LFS was delivered at –70 mV but not –40 mV. Moreover, DHPG (50 µM) was not able to induce chemical LTD, but when DCG IV (0.5 µM) was treated together, LTD was induced. These results indicate the involvement of Group I and II mGluR in the two type of perirhinal cortex LTDs (Fig. 1D).
These mGluRs are also necessary in familiarity discrimination [85]. When MPEP (3 mg/kg) or Group II mGluR antagonist LY 341495 (3 mg/kg) was systemically administered at the acquisition period, it had no effect on familiarity discrimination. However, when the two drugs were combined, familiarity discrimination was impaired at 24 h, but not at 15 min after drug administration. The drugs had no effect when it was treated after the sample phase of before the test. Thus, these results address the importance of Group I mGluR, especially mGluR5, and Group II mGluR in the acquisition.
The visual cortex is the region in the dorsal part of our brain responsible for visual information processing. It is one of the most well-investigated regions and is a popular subject for neuroscientific investigation [8687888990]. The visual cortex is composed of six layers and layer II/III and V are the major pyramidal cell layers [91]. Inhibitory interneurons are positioned in the layer II/III, IV and V, and it consists of PV, SOM, vasoactive intestinal peptide (VIP) and reelin positive intereneurons [92].
However, limited data are available on mGluR-dependent LTD in the visual cortex [93]. LTD was induced when quisqualate (10 µM), an agonist of the AMPAR, kainate receptor (KAR) and Group I mGluR, was introduced into the rat visual cortex in the presence of CNQX, AMPA and KAR antagonist, and APV [23]. Applying mGluR agonist trans-1-amino-cyclopentane-1,3-dicarboxylic acid (tACPD, 10 µM) itself had only an acute depression effect in layer II and III neurons of rat visual cortex slices; however, LTD was induced by the combined treatment with APV and tetanization [2324]. There was also a study examining the layer variations of LTD in the rat visual cortex using pairing protocols [26]. They treated LY 341495 (100 µM) in a high concentration to block all mGluRs and found that only LTD in layer VI neurons were mGluR-dependent with the pairing protocol. In mice, LFS-induced LTD was dependent on NMDAR and mGluR2 in layer II/III neurons [94]. LTD was induced by stimulation with 900 pulses of 1 Hz, but was blocked by treatment with APV (50 µM) or mGluR2 antagonist MCCG (100 µM, Fig. 1E). It was also impaired in mGluR2 KO mice. Chemical LTD was also induced with DCG IV (1 µM, 20 min).
However, the ocular dominance plasticity was normal in mGluR2 KO mice indicating the independence of mGluR2 dependent LTD in this process.
We have reviewed the mGluR-dependent LTDs in a variety of cortical regions of the brain. In addition, we provided an overview of the physiological conditions involved in this type of LTD and mGluR activation itself. The ACC and IC showed the relation of Group I mGluR-dependent LTD and pain related situations. Group II mGluR-dependent LTD was observed in the PFC and perirhinal cortex, which are involved in addiction and learning. LTD in the visual cortex was also dependent on Group II mGluR, but further investigations are necessary to determine the related functions.
Most of the reported and on-going studies on mGluR synaptic plasticity involve the role of the hippocampus, cerebellum, VTA, and striatum in learning and memory, motor function, and drug addiction fields. Neurodegenerative disease models are frequently used in these investigation. The cortex regions examined in this review have been investigated to a relatively lesser extent, and further investigations are necessary in relation to other specific behaviors or pathological situations. In addition, recent advances in pharmacology and technology are expected to facilitate studies on in vivo synaptic plasticity during such behaviors or specific conditions to identify solutions to more complex questions.
References
1. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008; 33:18–41. PMID: 17728696.

2. Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992; 89:4363–4367. PMID: 1350090.

3. Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992; 9:967–975. PMID: 1419003.

4. Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997; 18:969–982. PMID: 9208864.

5. Bashir ZI, Jane DE, Sunter DC, Watkins JC, Collingridge GL. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur J Pharmacol. 1993; 239:265–266. PMID: 8223907.

6. Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008; 65:2913–2923. PMID: 18712277.

8. Kano M, Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987; 325:276–279. PMID: 2880297.

9. Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982; 324:113–134. PMID: 7097592.

10. Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997; 17:7503–7522. PMID: 9295396.

11. Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997; 36:1517–1532. PMID: 9517422.

12. Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010; 11:459–473. PMID: 20559335.

13. Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007; 27:2958–2968. PMID: 17360919.

14. Otani S, Daniel H, Takita M, Crépel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002; 22:3434–3444. PMID: 11978820.

15. Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999; 38:495–504. PMID: 10221753.

16. Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001; 65:339–365. PMID: 11527572.

17. Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000; 288:1254–1257. PMID: 10818003.

18. Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006; 95:2427–2438. PMID: 16421200.

19. Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007; 27:11624–11634. PMID: 17959805.

20. Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci. 2012; 32:11318–11329. PMID: 22895715.

21. Liu MG, Koga K, Guo YY, Kang SJ, Collingridge GL, Kaang BK, Zhao MG, Zhuo M. Long-term depression of synaptic transmission in the adult mouse insular cortex in vitro. Eur J Neurosci. 2013; 38:3128–3145. PMID: 23930740.
22. Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci. 1999; 19:9346–9354. PMID: 10531439.

23. Kato N. Dependence of long-term depression on postsynaptic metabotropic glutamate receptors in visual cortex. Proc Natl Acad Sci U S A. 1993; 90:3650–3654. PMID: 8097320.

24. Kato N. Long-term depression requiring tACPD-receptor activation and NMDA-receptor blockade. Brain Res. 1994; 665:158–160. PMID: 7882011.

25. Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000; 3:150–156. PMID: 10649570.

26. Rao Y, Daw NW. Layer variations of long-term depression in rat visual cortex. J Neurophysiol. 2004; 92:2652–2658. PMID: 15212419.

27. Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, Collingridge GL. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001; 537:421–430. PMID: 11731575.
28. Kleppisch T, Voigt V, Allmann R, Offermanns S. G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent longterm depression but show normal long-term potentiation in the hippocampal CA1 region. J Neurosci. 2001; 21:4943–4948. PMID: 11438569.
29. Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptormediated long-term depression. J Neurosci. 2006; 26:2544–2554. PMID: 16510732.

30. Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001; 4:1079–1085. PMID: 11687813.

31. Steinberg JP, Huganir RL, Linden DJ. N-ethylmaleimide-sensitive factor is required for the synaptic incorporation and removal of AMPA receptors during cerebellar long-term depression. Proc Natl Acad Sci U S A. 2004; 101:18212–18216. PMID: 15608060.

32. Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000; 25:635–647. PMID: 10774731.

33. Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000; 3:1107–1112. PMID: 11036267.

34. Moult PR, Corrêa SA, Collingridge GL, Fitzjohn SM, Bashir ZI. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptordependent long-term depression. J Physiol. 2008; 586:2499–2510. PMID: 18356198.

35. Rush AM, Wu J, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci. 2002; 22:6121–6128. PMID: 12122073.
36. Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signalregulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004; 24:4859–4864. PMID: 15152046.

37. Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008; 59:70–83. PMID: 18614030.

38. Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008; 59:84–97. PMID: 18614031.

39. Moult PR, Schnabel R, Kilpatrick IC, Bashir ZI, Collingridge GL. Tyrosine dephosphorylation underlies DHPG-induced LTD. Neuropharmacology. 2002; 43:175–180. PMID: 12213271.

40. Schnabel R, Kilpatrick IC, Collingridge GL. An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology. 1999; 38:1585–1596. PMID: 10530820.

41. Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008; 28:10561–10566. PMID: 18923032.

42. Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Aktmammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004; 24:6352–6361. PMID: 15254091.

43. Gladding CM, Fitzjohn SM, Molnár E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009; 61:395–412. PMID: 19926678.

44. Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic longterm depression: mechanisms and implications for circuitry and disease. Neuron. 2010; 65:445–459. PMID: 20188650.

45. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000; 4:215–222. PMID: 10827444.

46. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005; 6:533–544. PMID: 15995724.

47. Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008; 31:199–207. PMID: 18329111.

48. Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006; 26:7555–7564. PMID: 16855083.

49. Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016; 17:485–496. PMID: 27307118.

50. Delevich K, Tucciarone J, Huang ZJ, Li B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci. 2015; 35:5743–5753. PMID: 25855185.

51. Wu LJ, Li X, Chen T, Ren M, Zhuo M. Characterization of intracortical synaptic connections in the mouse anterior cingulate cortex using dual patch clamp recording. Mol Brain. 2009; 2:32. PMID: 19828050.

52. Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004; 5:209–217. PMID: 14976520.

53. Escobar ML, Alcocer I, Chao V. The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo. Brain Res. 1998; 812:246–251. PMID: 9813352.

54. Gal-Ben-Ari S, Rosenblum K. Molecular mechanisms underlying memory consolidation of taste information in the cortex. Front Behav Neurosci. 2012; 5:87. PMID: 22319481.

55. Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007; 128:20–30. PMID: 17011704.

56. Mazzola L, Isnard J, Peyron R, Mauguière F. Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain. 2012; 135:631–640. PMID: 22036962.

57. Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005; 64:1175–1183. PMID: 15824343.

58. Liu MG, Kang SJ, Shi TY, Koga K, Zhang MM, Collingridge GL, Kaang BK, Zhuo M. Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings. J Neurophysiol. 2013; 110:505–521. PMID: 23636718.

59. Qiu S, Chen T, Koga K, Guo YY, Xu H, Song Q, Wang JJ, Descalzi G, Kaang BK, Luo JH, Zhuo M, Zhao MG. An increase in synaptic NMDA receptors in the insular cortex contributes to neuropathic pain. Sci Signal. 2013; 6:ra34. PMID: 23674822.

60. Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002; 5:573–579. PMID: 12006982.
61. Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tracttracing study in the rat. J Comp Neurol. 2004; 468:425–440. PMID: 14681935.

62. Reep RL, Winans SS. Afferent connections of dorsal and ventral agranular insular cortex in the hamster Mesocricetus auratus. Neuroscience. 1982; 7:1265–1288. PMID: 7110587.
63. Yamamoto K, Koyanagi Y, Koshikawa N, Kobayashi M. Postsynaptic cell type-dependent cholinergic regulation of GABAergic synaptic transmission in rat insular cortex. J Neurophysiol. 2010; 104:1933–1945. PMID: 20685921.

64. Liu MG, Zhuo M. Loss of long-term depression in the insular cortex after tail amputation in adult mice. Mol Pain. 2014; 10:1. PMID: 24398034.

65. Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998; 353:1819–1828. PMID: 9854254.
66. Lara AH, Wallis JD. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci. 2015; 9:173. PMID: 26733825.

67. Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2004; 2:e365. PMID: 15510225.

68. Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007; 315:1860–1862. PMID: 17395832.

69. Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002; 27:699–711. PMID: 12431845.

70. Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009; 192:489–497. PMID: 19030851.

71. Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000; 405:347–351. PMID: 10830963.

72. Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996; 351:1445–1453. PMID: 8941956.

73. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001; 24:167–202. PMID: 11283309.

74. Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011; 44:449–464. PMID: 22076606.

75. Otani S, Auclair N, Desce JM, Roisin MP, Crépel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999; 19:9788–9802. PMID: 10559388.

76. Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, Conn PJ. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A. 2015; 112:1196–1201. PMID: 25583490.

77. Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007; 30:99–122. PMID: 17417938.

78. Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007; 30:176–184. PMID: 17335914.

79. Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001; 2:51–61. PMID: 11253359.

80. Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998; 55:149–189. PMID: 9618747.

81. Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behav Neurosci. 1995; 109:613–619. PMID: 7576205.

82. Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993; 13:5418–5432. PMID: 8254384.

83. Watson C, Paxinos G, Puelles L. The mouse nervous system. 1st ed. Amsterdam, Boston: Elsevier Academic Press;2012. p. 795.
84. McCaffery B, Cho K, Bortolotto ZA, Aggleton JP, Brown MW, Conquet F, Collingridge GL, Bashir ZI. Synaptic depression induced by pharmacological activation of metabotropic glutamate receptors in the perirhinal cortex in vitro. Neuroscience. 1999; 93:977–984. PMID: 10473262.

85. Barker GR, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006; 13:178–186. PMID: 16585793.

86. Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012; 490:226–231. PMID: 23060193.

87. Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992; 356:150–152. PMID: 1545866.

88. Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989; 338:334–337. PMID: 2922061.

89. Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962; 160:106–154. PMID: 14449617.

90. Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013; 16:1068–1076. PMID: 23817549.

91. Laramée ME, Boire D. Visual cortical areas of the mouse: comparison of parcellation and network structure with primates. Front Neural Circuits. 2015; 8:149. PMID: 25620914.

92. van Versendaal D, Levelt CN. Inhibitory interneurons in visual cortical plasticity. Cell Mol Life Sci. 2016; 73:3677–3691. PMID: 27193323.

93. Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol. 2006; 78:17–37. PMID: 16423442.

94. Renger JJ, Hartman KN, Tsuchimoto Y, Yokoi M, Nakanishi S, Hensch TK. Experience-dependent plasticity without long-term depression by type 2 metabotropic glutamate receptors in developing visual cortex. Proc Natl Acad Sci U S A. 2002; 99:1041–1046. PMID: 11805343.

Fig. 1
Models of cortical mGluR-LTDs.
(A) mGluR LTD in the ACC is mGluR1, L-VGCC and partially NMDAR dependent. (B) mGluR LTD in the IC is mGluR5, NMDAR, L-VGCC, CB1R and PP1/2A dependent. (C) mGluR LTD in the PFC is Group II mGluR, PLC, IP3, PKC and NMDAR dependent. (D) mGluR LTD in the perirhinal cortex is mGluR5, Group II mGluR and NMDAR dependent. (E) mGluR LTD in the visual cortex is Group II mGluR and NMDAR dependent.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download