Abstract
Inositol-1,4,5-triphosphate [IP3] receptors binding protein released with IP3 (IRBIT) was previously reported as an activator of NBCe1-B. Recent studies have characterized IRBIT homologue S-Adenosylhomocysteine hydrolase-like 2 (AHCYL2). AHCYL2 is highly homologous to IRBIT (88%) and heteromerizes with IRBIT. The two important domains in the N-terminus of AHCYL2 are a PEST domain and a coiled-coil domain which are highly comparable to those in IRBIT. Therefore, in this study, we tried to identify the role of those domains in mouse AHCYL2 (Ahcyl2), and we succeeded in identifying PEST domain of Ahcyl2 as a regulation region for NBCe1-B activity. Site directed mutagenesis and coimmunoprecipitation assay showed that NBCe1-B binds to the N-terminal Ahcyl2-PEST domain, and its binding is determined by the phosphorylation of 4 critical serine residues (Ser151, Ser154, Ser157, and Ser160) in Ahcyl2 PEST domain. Also we revealed that 4 critical serine residues in Ahcyl2 PEST domain are indispensable for the activation of NBCe1-B using measurement of intracellular pH experiment. Thus, these results suggested that the NBCe1-B is interacted with 4 critical serine residues in Ahcyl2 PEST domain, which play an important role in intracellular pH regulation through NBCe1-B.
Fluid and HCO3– secretion is very important for the survival of many secretory epithelia. Aberrancy in this process results in tissue destruction, as that observed in cystic fibrosis (CF) and pancreatitis [12]. The pancreatic duct is an excellent model for studying epithelial fluid and HCO3– secretion because it secretes the bulk of fluid in pancreatic juice, does not absorb Na+, and secretes almost 140 mM HCO3– [3]. Ductal fluid and HCO3– secretion involves HCO3– entry into the basolateral membrane (BLM), which is largely mediated by Na+-HCO3– cotransporter isoform NBCe1-B [456], and its exit from the luminal membrane (LM), which is mediated by Cl–-HCO3– exchangers Solute Carrier Family 26 (Anion Exchanger), Member 3 (SLC26A3) and Solute Carrier Family 26 (Anion Exchanger), Member 6 (SLC26A6) that function along with CF transmembrane-conductance regulator (CFTR) [78910]. SLC26A3 and SLC26A6 (SLC26A3/6) are electrogenic with isoform-specific Cl–-HCO3– exchange stoichiometry [891011], that CFTR and SLC26A3/6 regulate each other [12], and established the importance of the interaction between CFTR and SLC26A3/6 in ductal fluid and HCO3– secretion [13].
IRBIT was originally discovered as a protein that inhibits the activation of IP3R [14] and competes with IP3 for binding to IP3 receptors for controlling Ca2+ signaling [1516]. IRBIT includes a PEST domain (amino acids 65~92), which contains 11 serine residues that can be phosphorylated, and coiled-coil (C-C) domain (amino acids 111~138) [1718]. IRBIT regulates NBCe1-B activity by binding to 40~62 residues in its N-terminus [19]. Phosphorylation of the PEST domain is essential for the interaction and activation of NBCe1-B by IRBIT [15]. IRBIT regulates NBCe1-B activity in the BLM and CFTR activity in the LM of pancreatic ducts, thus functioning as the key protein in epithelial fluid and HCO3– secretion [20]. Direct binding of the PEST domain of IRBIT to NBCe1-B increases NBCe1-B activity [20]. Phosphorylation of several serine residues in the PEST domain regulates the interaction between IRBIT and IP3R [16]. Mutations in Ser68, Ser71, Ser74, or Ser77 in the PEST domain of IRBIT decrease its ability to bind to and phosphorylate IP3R [16].
AHCYL2 (also known as KIAA0828 or long-IRBIT) is highly homologous to IRBIT and interacts with and regulates NBCe1-B similar to IRBIT [21]. The sequence of the N-terminus of AHCYL2 is highly similar to the N-terminus of IRBIT and contains protein phosphatase 1 (PP1)-binding site, PEST domain, C-C domain, and PDZ ligand. In addition, the N-terminus of AHCYL2 contains a long IRBIT-specific N-terminal (LISN) domain [17]. However, unlike IRBIT, AHCYL2 only weakly interacts with IP3R [16].
In this study, we used site-directed mutagenesis and coimmunoprecipitation assay to demonstrate that PEST domain of Ahcyl2 (gene name mKIAA0828) and 4 critical serine residues in the PEST domain of Ahcyl2 are essential for specific binding of NBCe1-B. A measurement of intracellular pH experiment also revealed that phosphorylation of the 4 critical serine residues in Ahcyl2 PEST domain is required for activation of NBCe1-B. In summary, our results show that Ahcyl2 upregulates NBCe1-B via 4 critical serine residues of the PEST domain-mediated association and is likely to be another regulator of HCO3– secretion.
NBCe1-B constructs used in this study have been described previously [20]. A cDNA clone of Ahcyl2 was purchased from Open Biosystems (GE Healthcare, Huntsville, AL, USA). The cDNA encoding full-length Ahcyl2 was subcloned into p3xFLAG-CMV-7.1 mammalian expression vector (Sigma-Aldrich, St. Louis, MO, USA) to generate FLAG-Ahcyl2. Point and deletion mutagenesis were induced by performing site-directed mutagenesis with the QuikChange mutagenesis kit (Stratagene, La Jolla, CA, USA). PEST domain deletion mutant (deletion of amino acids 148~175) and C-C domain deletion mutant (deletion of amino acids 194-231) were constructed by PCR splicing using the full-length Ahcyl2 gene in the p3xFLAG-CMV- 7.1 vector as template. PCR was performed by using primer to delete PEST and C-C domain region, 5'-GGA CGT CGC TCT TTG TCT GAC AAG CAG CAA AAG AAC -3' (PEST domain deletion mutant) 5'-TTC TGT GTC AAG AAC ATC GTG GGT TGT ACG CAC ATC-3' (C-C domain deletion mutant) that encompassed the deletion. All the constructs were verified by sequencing their entire open reading frames. Standard bath solution was prepared using 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES (pH 7.4 with NaOH), and 10 mM glucose. Na+-free solution was prepared by replacing Na+ with N-methyl-d-glucamine. HCO3–-buffered solution was prepared by replacing 25 mM Na+ salts with 25 mM Na+-HCO3– and by reducing the concentration of HEPES to 2.5 mM and was gassed using 5% CO2 and 95% O2. Osmolarity of all the solutions was adjusted to 310 mOsm by using a major salt. Anti-GFP and anti-FLAG antibodies for performing coimmunoprecipitation experiment were purchased from Invitrogen (Carlsbad, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively.
All transfections (HeLa and HEK293T cells) were performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Preliminary experiments were performed to determine optimal transfection condition. When cells were transfected with multiple plasmids, transfection ratio was set in preliminary experiments and the total number of plasmids was kept constant for all conditions by using an empty vector. Single transfected cells were identified and were cotransfected with GFP. GFP fluorescence intensity was used to select cells showing similar expression level of cotransfected plasmid.
Intracellular pH (pHi) was measured by determining the fluorescence of BCECF-AM (Teflab, Austin, TX, USA) at excitation wavelengths 490 and 440 nm and by calibrating fluorescence signals as indicated previously [20]. HeLa cells were used for intracellular pH measurements, because HeLa cells are strongly adherent (i.e. they stick tightly to the coverslips) to endure the fast flow rates. Fluorescence was recorded using clusters of 2~5 transfected HeLa cells [20]. HeLa cells loaded with BCECF-AM were perfused with HEPES-buffered medium for at least 5 min before measuring pHi. Na+-HCO3– cotransport was induced through acid loading by perfusing Na+-free HCO3–-buffered medium containing 10 µM S -(N-ethyl-N-isopropyl) amiloride (EIPA), which blocks Na+/H+ exchange. The cells were then perfused with HCO3–-buffered medium containing 140 mM Na+. NBCe1-B activity is initiated by perfusing the HeLa cells with HCO3–-buffered medium containing 140 mM Na+. The rates of Na+-dependent changes in pHi (ΔpHi(recovery)/ΔpHi(total)) reflect NBCe1-B activity. Also, NBCe1-B activity is determined from the slopes of the first derivatives of beginning 30~45 sec of pHi increase and is given as ΔpHi/min.
We performed co-immunoprecipitation assays with HEK293T cells because of high-yield transfection efficiency in these cells. HEK293T cell extracts were prepared by disrupting the cells in ice-cold lysis buffer (20 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail), by incubating the cells for 15~30 min, followed by centrifugation (20,000xg, 30 min). After protein quantification using the DC Protein Assay (Bio-Rad, Hercules, CA, USA), the extracts containing 500 µg protein were then incubated with 1 µg of anti-GFP or anti-FLAG antibody beads. Next, antibody beads were collected and washed 3 times with lysis buffer. Proteins bound to the beads were recovered after heating the beads in 25 µl of SDS sample buffer at 65℃ for 30 min. The extracts, 1% (3 µg) of the input and 80% (20 µl ) of the immunoprecipitates were loaded, separated by 10% SDS-PAGE, transferred to PVDF membrane (Bio-Rad, Hercules, CA, USA), and detected with HRP-conjugated anti-GFP or anti-FLAG antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Western blot band intensity was determined and quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Comparison of amino acid sequences of Ahcyl2 and mIRBIT (mouse IRBIT; GenBank® accession number AB092504) showed that the structure of Ahcyl2 was similar to that of mIRBIT. However, Ahcyl2 had a longer N-terminus with an N-terminal appendage (residues 1~109), which was not present in mIRBIT. Amino acids of mIRBIT (Ser68, Ser71, Ser74, and Ser77) that are essential for interaction with NBCe1-B were determined by performing site-directed mutagenesis [15]. These amino acids were completely conserved in Ahcyl2 (Fig. 1), suggesting that Ahcyl2 interacted with NBCe1-B. We mutated 4 serine residues (Ser151, Ser154, Ser157, and Ser160) in the PEST domain of Ahcyl2, which corresponded to 4 serine residues (Ser68, Ser71, Ser74, and Ser77) in the PEST domain of mIRBIT, to alanine. In addition, we mutated the possibly essential Ser149 and Thr172 residues, which are not present in mIRBIT, to alanine (Fig. 1). Further, we mutated 4 amino acids (Glu208, Pro210, Met213, and Ala214) in the in C-C domain of Ahcyl2, which are not present in the C-C domain of mIRBIT, to equivalent amino acids present in mIRBIT (Fig. 1). Site-directed mutagenesis was performed for substituting Ser149 and Thr172 in Ahcyl2 with alanine, and deletion mutagenesis was performed for substituting the specified amino acids in the PEST and C-C domains of Ahcyl2 with alanine (Fig. 1).
Interaction between IRBIT and NBCe1-B involves the phosphorylation of IRBIT [15]. The N-terminal domain of IRBIT contains a serine-/threonine-rich region that is important for interaction with NBCe1-B [15]. To determine whether phosphorylation of the serine-/threonine-rich region of Ahcyl2 was required for its interaction with NBCe1-B, we examined the interaction of Ahcyl2 mutants that changed to be equivalent amino acids from 4 amino acids (E209D, P211S, M214I, A215S) and Ahcyl2 C-C domain deletion mutant with NBCe1-B by performing coimmunoprecipitation (Fig. 2A). All the Ahcyl2 mutants could bind to NBCe1-B in a similar manner as wild-type Ahcyl2 (Fig. 2A). These results indicated that the 4 amino acids namely, Glu208, Pro210, Met213, and Ala214 in Ahcyl2, which are not present in mIRBIT, and the C-C domain of Ahcyl2 were not involved in the interaction of Ahcyl2 with NBCe1-B (Fig. 2B). Next, we examined whether the PEST domain of Ahcyl2 was involved in the interaction between Ahcyl2 and NBCe1-B. 4 serine residues in the PEST domain of mIRBIT are similar to those present in the PEST domain of Ahcyl2. We generated mutation of 4 serine residues as well as Ser149 and Thr172 not existing in mIRBIT into alanine and PEST domain deletion mutant. We observed that NBCe1-B did not interact with the Ahcyl2 PEST domain deletion mutant (Fig. 2C), indicating that the PEST domain was important for the interaction of Ahcyl2 with NBCe1-B. The 4 substitution mutants (S151A, S154A, S157A, and S160A) provided the same results as the PEST domain deletion mutant (Fig. 2C). These results indicated that the 4 serine residues in the PEST domain of Ahcyl2 and phosphorylation of Ahcyl2 were important for its interaction with NBCe1-B. Binding of the 2 substitution mutants (S149A, and T172A) to NBCe1-B was similar to that observed for the C-C domain of Ahcyl2 (Fig. 2D).
HCO3– influx across the BLM is needed for NBCe1-B-mediated fluid and HCO3– secretion in various species, including humans, guinea pigs, and mice [9]. IRBIT remarkably activates NBCe1-B [1520]. To compare the activation of NBCe1-B by mIRBIT and Ahcyl2, we measured the effect of mIRBIT and Ahcyl2 on NBCe1-B transporter activity in HeLa cells. NBCe1-B activity depends on Na+ and HCO3–, and Anion exchanger inhibitor, 4,4'-Diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS) inhibits the recovery of acid load in the presence of EIPA, the blocker of endogenous Na+-H+ exchanger [20]. It seems that Ahcy2 is similar with mIRBIT (Fig. 3A~3C), indicating that Ahcyl2 also regulated NBCe1-B (Fig. 3D). Next, we measured the changes in pHi in HeLa cells coexpressing Ahcyl2 mutants with NBCe1-B to determine whether the C-C domain of Ahcyl2 was involved in the regulation of NBCe1-B activity. Ahcyl2 mutants E209D, P211S, M214I, and A215S, which contained mutated amino acids in the C-C domain, were not similar to mIRBIT containing the corresponding amino acids. We observed that all these mutants increased NBCe1-B activity similar to that by wild-type Ahcyl2 (Fig. 4A~4G). This result suggested that the 4 amino acids, namely, Glu208, Pro210, Met213, and Ala214 which are not present in mIRBIT, and the C-C domain of Ahcyl2 did not affect NBCe1-B activity (Fig. 4H).
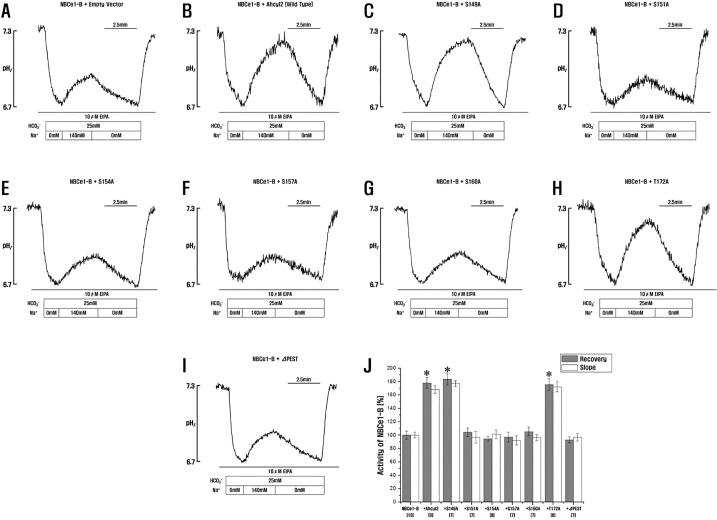
The mIRBIT contains several domains including a PEST domain, a C-C Domain and a AHCY domain (also termed the AHCY-like domain) [18]. The PEST domain of IRBIT, which contains 16 serine residues, is a very important functional domain. Phosphorylation of Ser68, Ser71, Ser74, and Ser77 of mIRBIT is required for binding to IP3Rs [14] and for binding to and activation of NBCe1-B [15]. Therefore, we examined the effect of the Ahcyl2 mutants on NBCe1-B activity. Changes in pHi indicated that NBCe1-B activity in cells transfected with vector expressing the Ahcyl2 PEST domain deletion mutant was similar to that in cells transfected with an empty vector (Fig. 5A and 5I). This interesting result indicated that the PEST domain of Ahcyl2 affected NBCe1-B activity. Moreover, the finding that the 4 substitution mutants (S151A, S154A, S157A, and S160A) exerted a similar effect on NBCe1-B activity as the PEST domain deletion mutant indicated the 4 serine residues (Ser151, Ser154, Ser157, and Ser160) of Ahcyl2 were important for altering NBCe1-B activity (Fig. 5D, 5E, 5F, 5G, 5I and 5J). The 2 substitution mutants (S149A, and T172A) and the Ahcyl2 C-C domain deletion mutant exerted similar effects on NBCe1-B activity (Fig. 4G, 5C and 5H).
AHCYL2 contains an N-terminal extension called the LISN domain, which is rich in proline and alanine residues. Overexpressed AHCYL2 shows weak affinity toward IP3R, and endogenous AHCYL2 expressed in the cerebellum does not bind to IP3R. Deletion mutagenesis indicated that the LISN domain was essential for inhibiting the interaction of AHCYL2 with IP3R [22]. Low affinity of AHCYL2 toward IP3R suggests that AHCYL2 and IRBIT play different roles in fluid and HCO3– secretion by secretory epithelia. Expression of AHCYL2 is ubiquitous, with high levels being expressed in the cerebrum, cerebellum, kidneys, and ovaries and low levels being expressed in the liver and lungs [22]. However, expression of AHCYL2 is much lower than that of IRBIT in most tissues in which they are coexpressed [22], suggesting that their functions are not cooperative but rather distinct. This is more obvious in their cell-specific expression in the brain. AHCYL2 is expressed at high levels mostly in cerebellar molecular layer interneurons, whereas IRBIT is usually expressed in glial cells [22]. IRBIT and AHCYL2 form a heteromultimer [22]. Because the C-terminal region of IRBIT, which is involved in its multimerization [16], is conserved in AHCYL2, AHCYL2 binds to IRBIT through the C-terminal region. IRBIT and AHCYL2 may not interact with IP3R in their heteromultimeric form [1622]. AHCYL2 weakly inhibits the interaction between IRBIT and IP3R and counteracts IRBIT-mediated suppression of IP3-induced Ca2+ release [22]. AHCYL2 undergoes 2 types of phosphorylations. One type of phosphorylation occurs in the conserved serine-rich region, which is similar to that occurring in IRBIT. The other type of phosphorylation depends on the LISN domain and occurs during neural differentiation [22].
Recently, it was reported that AHCYL2 regulates intracellular Mg2+ sensitivity during the inhibition of NBCe1-B currents in a specific intracellular ionic condition and binds to the cytosolic N-terminus of NBCe1-B in bovine parotid acinar (BPA) cells [21]. However, the role of the AHCYL2 domains on the interaction with NBCe1-B and activation of NBCe1-B have not been revealed. Therefore, in this study, we investigated the interaction and regulation between the important domains of Ahcyl2, i.e., the PEST and C-C domains, with NBCe1-B. Deletion of the C-C domain was not enough to reduce their binding affinity to NBCe1-B (Fig. 2A and 2B). As shown in Fig. 2C and 2D, deletion of the PEST domain was sufficient to inhibit interaction of Ahcyl2 with NBCe1-B. We found that phosphorylation of multiple serine residues (Ser151, Ser154, Ser157, and Ser160) in the Ahcyl2 PEST domain was essential not only for promoting the interaction of Ahcyl2 with NBCe1-B but also for regulating NBCe1-B activity, which was similar to that observed for the interaction between IRBIT and NBCe1-B. Therefore, it can be concluded that Ahcyl2 binds to NBCe1-B and regulates its activity in a manner similar to that by IRBIT. Phosphorylation of IRBIT induces its translocation from cytosol to membrane, through the interaction with IP3R [22] and NBCe1-B [2023]. Explaining phosphorylation state of IRBIT in secretory epithelia and physiological functions are expected to reveal mechanisms of the regulation of NBCe1-B activity by phosphorylation of IRBIT.
We suggested that Ahcyl2 increases NBCe1-B activity via the Ahcyl2 PEST domain in vitro. However, role of Ahcyl2 in regulating NBCe1-B activity in vivo is still unknown. It should be determined using an Ahcyl2 knockdown system. Epithelial HCO3– secretion begins with the entry of HCO3– into the BLM, which is mediated by NBCe1-B, and its exit from the LM, which is mediated by the coordinated activity of Cl– channel CFTR and Cl–-HCO3–exchanger SLC26. SLC26 controls Cl– absorption and HCO3– secretion and CFTR regulates Cl– efflux for maintaining HCO3– secretion [1013]. HCO3– serves to control the pH of bodily fluids and, most importantly, HCO3– facilitates solubilization of ions and macromolecules, like mucins and digestive enzymes, in secreted fluids. When this process fails, even partially, the tissues are destroyed, as happens to the pancreas, the ductus deferens (or vas deferens) and the lung epithelium in CF [1]. Therefore, understanding HCO3– secretion and its alteration in CF may lead to development of new modalities to halt progression of the disease. IRBIT regulates CFTR activation by increasing CFTR open probability [20]. Interestingly, although IRBIT-induced CFTR activation requires the phosphorylation of Ser68 [20], the pattern of interaction between IRBIT and CFTR is different from that between IRBIT and NBCe1-B. Therefore, deletion of the PEST or C-C domain or the PDZ ligand of IRBIT does not inhibit the interaction between IRBIT and CFTR. The results of the present study showed that Ahcyl2 regulated NBCe1-B activity in a similar manner as IRBIT. However, further studies should be performed to investigate the interaction between Ahcyl2 and CFTR. Results of these studies will help clarify the role of Ahcyl2 as a regulator of epithelial HCO3– secretion. If the relationship between Ahcyl2 and CFTR is similar to that between IRBIT and CFTR, it would be important to determine the role of the Ahcyl2 LISN domain in this interaction. In summary, we identified NBCe1-B as the target molecule of Ahcyl2 and found that Ahcyl2 PEST domain significantly regulated pHi through NBCe1-B. Results of this study would help in clarifying the role of Ahcyl2 domains in epithelial HCO3– secretion. Further studies including the relationship between Ahcyl2 and CFTR and the role of Ahcyl2 as a regulator of epithelial HCO3– secretion will be necessary to explain the physiological function of Ahcyl2.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation of Korea funded by the Korean Government (NRF-2014R1A1A1A05002735).
References
1. Durie PR. The pathophysiology of the pancreatic defect in cystic fibrosis. Acta Paediatr Scand Suppl. 1989; 363:41–44. PMID: 2701923.

2. Baron JH. The pancreas. Mt Sinai J Med. 2000; 67:68–75. PMID: 10677785.
3. Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005; 67:377–409. PMID: 15709963.

4. Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3– by Na+-HCO3– cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996; 495:169–178. PMID: 8866360.
5. Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3– transporters in the rat pancreatic duct. J Gen Physiol. 1994; 104:57–85. PMID: 7964596.
6. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998; 273:17689–17695. PMID: 9651366.

7. Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3– transport in mutations associated with cystic fibrosis. Nature. 2001; 410:94–97. PMID: 11242048.
8. Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3– transport in cystic fibrosis. EMBO J. 2002; 21:5662–5672. PMID: 12411484.
9. Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl–/HCO3– exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999; 274:14670–14677. PMID: 10329661.
10. Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004; 6:343–350. PMID: 15048129.

11. Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006; 273:177–186. discussion 186-192, 261-264. PMID: 17120768.

12. Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity
in vivo to determine pancreatic duct HCO3– secretion: relevance to cystic fibrosis. EMBO J. 2006; 25:5049–5057. PMID: 17053783.
13. Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda). 2008; 23:104–114. PMID: 18400693.

14. Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003; 278:10602–10612. PMID: 12525476.
15. Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3– cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A. 2006; 103:9542–9547. PMID: 16769890.
16. Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006; 22:795–806. PMID: 16793548.
17. Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J. 2007; 407:303–311. PMID: 17635105.

18. Devogelaere B, Sammels E, De Smedt H. The IRBIT domain adds new functions to the AHCY family. Bioessays. 2008; 30:642–652. PMID: 18536033.

19. Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3– cotransporters family. Proc Natl Acad Sci U S A. 2013; 110:4105–4110. PMID: 23431199.
20. Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3– secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009; 119:193–202. PMID: 19033647.
21. Yamaguchi S, Ishikawa T. AHCYL2 (long-IRBIT) as a potential regulator of the electrogenic Na+-HCO3– cotransporter NBCe1-B. FEBS Lett. 2014; 588:672–677. PMID: 24472682.
22. Ando H, Mizutani A, Mikoshiba K. An IRBIT homologue lacks binding activity to inositol 1,4,5-trisphosphate receptor due to the unique N-terminal appendage. J Neurochem. 2009; 109:539–550. PMID: 19220705.

23. Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011; 121:956–965. PMID: 21317537.

Fig. 1
Sequence alignment of mIRBIT and Ahcyl2.
Amino acids essential for interaction with NBCe1-B, as determined by performing site-directed and deletion mutagenesis, are indicated using upward-pointing arrows and bold bars. The PEST and C-C domains are shown by using bold bars.
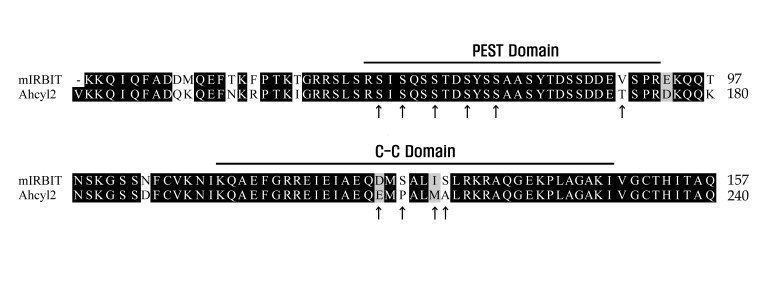
Fig. 2
Ahcyl2 interacts with specific domains and amino acids of NBCe1-B.
(A and C) HEK293T cells were transfected with GFP-NBCe1-B and an empty vector or vectors expressing FLAG-Ahcyl2, FLAG-point, and FLAG-deletion mutants. Interaction of Ahcyl2 with GFP–NBCe1-B was determined by performing coimmunoprecipitation assay. NBCe1-B was detected using anti-GFP antibody, and Ahcyl2 was detected using anti-FLAG antibody. (B and D) Densitometry analysis of efficiency of association of Ahcyl2 with NBce1-B expressed as amount of Ahcyl2 co-precipitates divided by the total amount of Ahcyl2 input [Ahcyl2-Blot/Ahcyl2-Input] (for comparison, results were normalized to 100% for Ahcyl2). (*p<0.01 versus Wild Type, n=3).
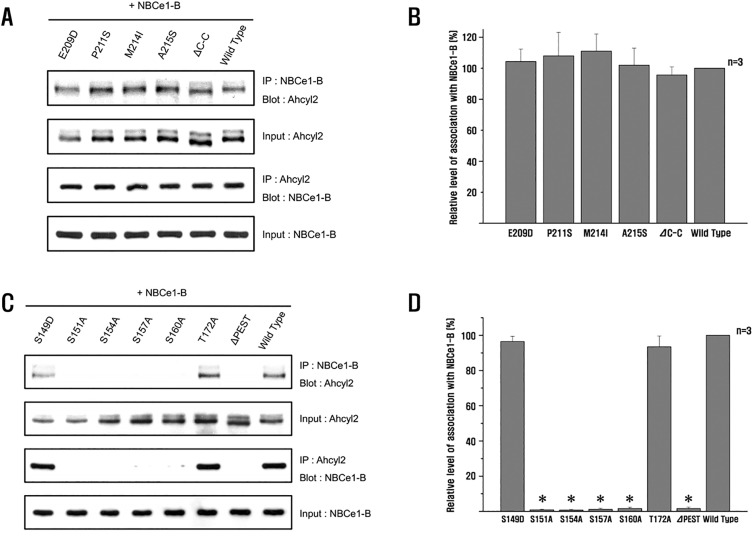
Fig. 3
Ahcyl2 stimulates NBCe1-B activity.
(A~C) HeLa cells were transfected with GFP-NBCe1-B and an empty vector or vectors expressing mIRBIT or Ahcyl2. (D) NBCe1-B activity in transfected HeLa cells was determined by measuring Na+-dependent recovery of acid load in the presence of 10 µM EIPA and normalized relative to control. In all, 9~10 experiments were performed for each condition. Numbers below the labels on the x-axis indicate the frequency of experiments. *p<0.01 versus NBCe1-B.
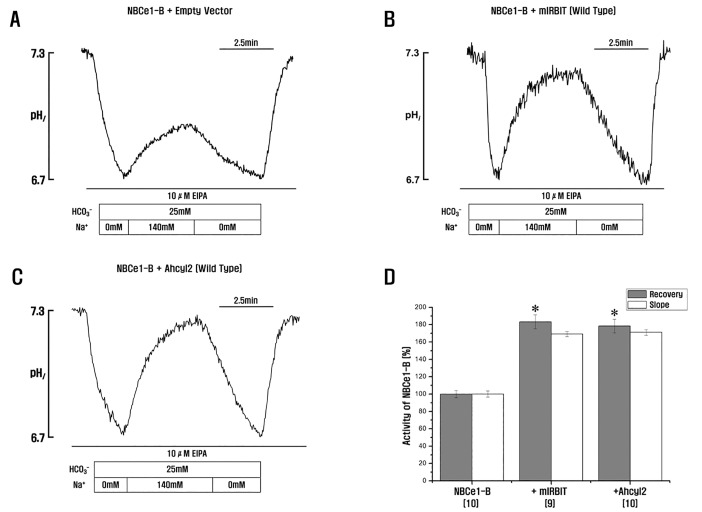
Fig. 4
Role of Ahcyl2 C-C domains in NBCe1-B activation.
(A~G) HeLa cells were transfected with GFP-NBCe1-B and an empty vector or vectors expressing Ahcyl2 or its point or C-C domain deletion mutant, (H) NBCe1-B activity was measured and normalized relative to control. Bars indicate mean±SEM of 7~10 separate experiments. *p<0.01 versus NBCe1-B. Numbers below the labels on the x-axis indicate the frequency of experiments.
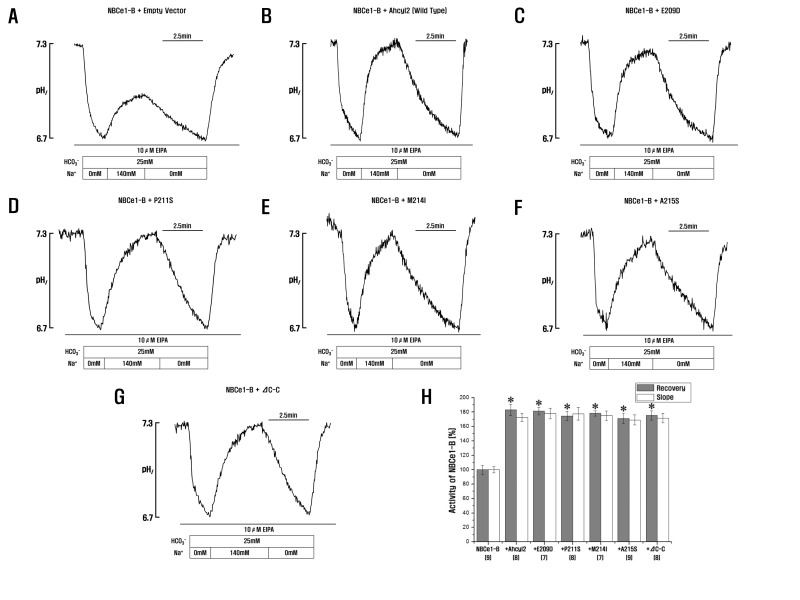
Fig. 5
Role of Ahcyl2 PEST domains in NBCe1-B activation.
(A~I) HeLa cells were transfected with GFP-NBCe1-B and an empty vector or vectors expressing Ahcyl2 or its point or PEST domain deletion mutant, (J) NBCe1-B activity was measured and normalized relative to control. Bars indicate mean±SEM of 7~10 separate experiments. *p<0.01 versus NBCe1-B. Numbers below the labels on the x-axis indicate the frequen cy of experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download