Abstract
Membrane-bound HLA-G (mHLA-G) discovery on adipose derived stem cells (ADSCs) as a tolerogenic and immunosuppressive molecule was very important. Many documents have shown that HLA-G expression can be controlled via some hormones such as progesterone (P4) and estradiol (E2). Therefore, this study was designed to evaluate progesterone and estradiol effects on mHLA-G in ADSCs at restricted and combination concentrations. Three independent cell lines were cultured in complete free phenol red DMEM and subcultured to achieve suffi cient cells. These cells were treated with P4, E2 and P4 plus E2 at physiologic and pregnancy concentrations for 3 days in cell culture conditions. The HLA-G positive ADSCs was measured via monoclonal anti HLA-G-FITC/MEMG-09 by means of flow cytometry in nine groups. Data were analyzed by one way ANOVA and Tukey's post hoc tests. There were no signifi cant values of the mean percentage of HLA-G positive cells in E2-treated and the combination of P4 plus E2-treated ADSCs compared to control cells (p value>0.05) but P4 had a signifi cant increase on mHLA-G in ADSCs (p value<0.05). High P4 concentration increased mHLA-G but E2 and the combination of P4 plus E2 could not change mHLA-G on ADSCs.
In the last decade, adult mesenchymal stem cells (MSCs) have produced a lot of intentions for the therapeutic purposes in different fields [12]. MSCs can be driven from many sources. Adipose derived stem cells (ADSCs) -one type of MSCs- can be obtained from healthy donor liposuction easily and they have low antigenicity and tri-lineage differentiation capacity which can be considered as candidates in tissue engineering, treatment of autoimmune and degenerative diseases as well as in reconstructive transplantation [3]. Nowadays, numerous researches have focused on intrinsic inhibitory properties of ADSCs. Immunosuppressive effect of ADSCs is comparing with bone marrow mesenchymal stem cells to participate in cell therapies [4]. Immunosuppressive effects of ADSCs are mediated by several factors (e.g. Prostaglandin E2 (PGE2), Indolamine 2, 3-dioxygenase enzyme (IDO), HLA-G (Human leukocyte antigen-G) and so on [56].
Indeed, HLA-G discovery as a tolerogenic molecule in ADSCs with endocrine and paracrine effects would be a therapeutic goal to improve autoimmune diseases, accept solid allografts and treat spontaneous recurrent abortions [5]. HLA-G has two forms: soluble and membrane-bound with the same immunomodulatory effects in environment [7]. Overall, membrane-bound HLA-G (mHLA-G) directs inhibitory mechanism through interaction with three well-known inhibitory receptors: ILT2 (Immunoglobulin-Like Transcript), ILT4 and KIR2DL4 (Killer Cell Immunoglobulin-Like Receptor) on monocyte, MQ (macrophage), B, T and NK (Natural Killer) cells and so suppress antigen-specific TCD8 and NK cytotoxic activity. Also, HLA-G can induce regulatory T cells and TH2 cytokine profiles. HLA-G gene encompasses regulatory sites in 3' UTR and 5' UTR. Many of cytokines, hormones and drugs impress on 5' UTR site. P4 can bind to PRE (Progesterone Response Element) site in the HLA-G promoter between positions -52 and -38 where overlaps the HLA-G TATA box and induce HLA-G expression [789] whereas miR-148a, miR-148b and miR-152 can down-regulate HLA-G expression by binding to the 3' UTR HLA-G mRNA [10111213].
Progesterone (P4) and estradiol (E2), as two essential hormones in pregnancy, play vital roles in decidual and placenta growth as well as fetus and maternal protection. In course of pregnancy, high values of P4 and E2 in maternal blood may enhance HLA-G expression from subpopulation including decidual trophoblast cells, amnion epitheliums, decidual stem cells and etc. ADSCs may be a probable supplement for soluble HLA-G detected in maternal blood. Soluble HLA-G is released by shedding mHLA-G into blood which participates in restraining uNKs (uterine Natural killer), TCD8, MQ and B cells to guard fetus as semiallograft tissue of invasive cells [14].
Progesterone can enhance immunomodulatory ability via up-regulation PGE2 and IL-6 [15]. Estradiol stimulates MSCs proliferation via HIF-1α(Hypoxia-Inducible Factor) activation and VEGF (Vascular Endothelial Growth Factor) expression [16] and enhances ADSCs efficacy in remyelination in mouse model of sclerosis [17].
It has been proved that P4 increases HLA-G expression but little is known about the E2 effect on mHLA-G in ADSCs. Also, recent documents have shown that estradiol can increase progesterone receptors (PgR) and estrogen receptors (ER) on some cells [1819]. Therefore, we proposed that the combination of P4 plus E2 may induce a more effect in HLA-G excitation. So, this study was programmed to evaluate E2 and P4 effects only and synergistic effects of E2 and P4 on mHLA-G levels in ADSCs in vitro.
All experiments were repeated with three independent ADSCs cell lines. The cryovials containing frozen ADSCs were kindly dedicated by Anatomical Science and Molecular Biology Department, Isfahan University of Medical Science. These cells had been confirmed in previous study so that, they were characterized for positive markers: CD105, CD90, CD44 and negative markers: CD14, CD45 by flow cytometery method [20].
ADSCs were cultured in 75 cm3 flasks in complete free phenol red low glucose DMEM (Dulbecco's Modified Eagle Medium) medium (Sigma) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Roche) and incubated under conditions 5% CO2, 95% humidity and at 37℃ temperature. Approximately, every three days, cell culture medium was changed. After one week, these cells reached to more than 80% conflueny and then were detached by 0.05% trypsin (Sigma) and 0.5 mM EDTA within 7 min, at 37℃ and again subcultured for more passages.
The cells were trypsinized, washed in Phosphate-Buffer Saline (PBS), pH 7.4 at 1500 rpm for 7 min then, counted by hemocytometer. The concentrations of 10–7 M, 10–9 M E2 and the concentrations of 1×10–5 M, 3×10–5 M P4 as well as the combination of E2:10–7 M+P4:1×10–5 M, E2:10–7 M+P4:3×10-5 M, E2:10–9 M+P4:1×10–5 M and E2:10–9 M+P4:3×10–5 M were prepared in finally 1 ml complete free phenol red DMEM. 105cells seeded in each well of 24-well plates. Then complete DMEM containing P4, E2 and the combinations of P4 plus E2 were separately infused into wells. Each of the concentrations were repeated in triplication and considered as treated groups. Additionally, some of the wells were just feed with 1ml complete DMEM without drugs as untreated cells (control group). These plates were kept for 3 days in cell culture incubator.
After this period, the cells were swept from the bottom of the wells by trypsin and washed in PBS. Treated cells and control cells were suspended in 1 ml PBS and then, 1 µL of monoclonal anti HLA-G-FITC/MEMG-09 (Exbio) were added into tubes. These tubes were incubated for 30 min in the dark and at 4℃. Also, isotypic control cell was prepared by adding 1 µL mouse IgG1 isotype control-FITC (Exbio) to untreated cell suspension in 1 ml PBS to confirm the specificity of primary antibody binding. After twice washing by wash solution, ADSCs were ready for analyzing at FACS Calibour flow cytometer (BD) and 10000 events were counted and then the gating of wanted cell population eliminated dead cells and debris. In the next step, the fluorescence intensity of gated cells was analyzed by Cell Quest Software program.
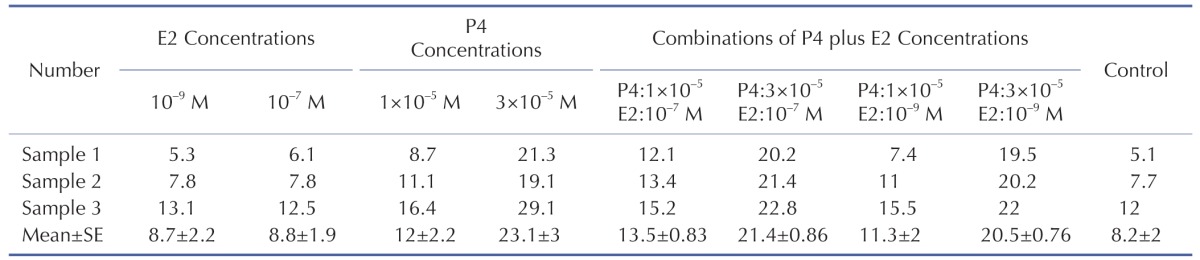
Isotype control antibody had no specificity for target cells (Fig. 1A) and the mean percentage of HLA-G positive ADSCs in control group was 8.2±2% (Fig. 1B).
The mean percentage of ADSCs with HLA-G expression in the vicinity of P4 increased from 12±2.2% at low P4 concentration (p>0.05) up to 23.1±3% at high P4 concentration (***p<0.05) compare to control group (Fig. 2).
The mean percentage of HLA-G positive cells treated with 10–9 M and 10–7 M E2 were found 8.7±2.2% and 8.8±1.9% respectively and had no significant increase compare to control group (p>0.05) (Fig. 3).
Flow cytometry graphs and statistical analysis of P4 plus E2-treated ADSCs are summarized in Fig. 4A~D, E. We expected that the P4 plus E2 combinations could induce a more increase in the HLA-G positive cells in comparison with P4-treated cells as control group but there were no significant increase in the mean percentage HLA-G positive cells (p>0.05).
Obtained percentages of all three cell lines before and after treatment with P4, E2 and P4 plus E2 concentration are summarized in Table 1.
To our knowledge, P4 is a lipid soluble hormone which can easily pass across membrane cell and its effects are mediated through PgR in the cytoplasm then receptors dimerization take place and these complexes enter to the nucleus and bind to PRE region in promoter target genes [21].
For the first time, Yei et al. showed that progesterone can enhance HLA-G gene expression in human cytotrophoblasts and JEG-3 cells (human placenta choriocarcinoma cell line). Also they verified that HLA-G molecule increased via western blot method [22].
One year later, Nasef et al. reported that approximately 13.7±1.3% of cultured BM-MSCs after 72h were HLA-G positive cells by using mHLA-G staining [23]. In the present study, we found 8.2±2% of untreated ADSCs were HLA-G positive. This document shows that the percentage of HLA-G positive ADSCs may be lower than BM-MSCs.
Blanco et al. observed that 14% of DSC (Desidoal stromal cells) cultured in opti-DMEM expressed HLA-G antigen. The intensity of HLA-G expression among the different DCS lines varied from 2 to 20%. In the second part, the effect of progesterone plus cAMP was quantified on extracellular HLA-G and mHLA-G increased up 47% within 15 days [24]. In our experiment, the range of HLA-G positive untreated cells varied from 5 to 12% and the P4-treated cells increased up 23% within 3 days.
In a study by Sheshgiri, smooth muscles and epithelial cells in culture medium did not express HLA-G molecule at baseline but it was induced after incubation with high dose of progesterone (1000 ng/ml) for 24 hr [25].
Ivanova-Todorova et al. reported that the percentage of HLA-G positive cells in cultured BM-MSCs, ADSCs and DSCs with 30 µM PgAC (Medroxy progesterone-17-acetate) after 72 h were 10.2, 11.4 and 5.3% respectively. In addition to flow cytometry, they confirmed their results by immunocytochemistry and western blot method. In mentioned study, higher amounts of ADSCs expressed extracellular HLA-G than BM-MSC [26]. On the other hand, cell culture conditions, incubation time and dose of drug in our experiment were similar to Ivanova but, 23% of ADSCs relieved HLA-G antigen after treatment with P4. It is probable due to exposure to pure P4. Still, the precise mechanism remains to be clarified.
E2 mechanism on HLA-G expression can be direct and indirect. Yun et al. have been mentioned that E2 can induce HIF-1α factor [16]. Hypoxia is an important factor which can involve in induction of HLA-G transcription via HIF-1α [27]. A hypoxia response element (HRE) site is located on promoter HLA-G between the –242 and –238 positions [78]. However, HRE function has not been found but it might mediate the induction of HLA-G expression via HIF-1α to HRE site binding.
In a study by He et al. estradiol/progesterone effects were examined on MCF-7 cells and at low concentrations of E2: 10, 100 ng/ml, MCF-7 cells did not submit significant value of HLA-G but at high dose of E2: 1000 ng/ml on MCF-7, these cells proposed HLA-G protein significantly. HLA-G expression on treated MCF-7 with P4 at 0.1 µg/ml was not significant but at 1, 10 µg/ml P4 was significant [28]. These results at P4 concentrations as well as low concentration of E2 had harmony with our results but we did not measure HLA-G positive cells at higher concentration of E2 because we mimicked of E2 concentration in pregnancy.
Tao et al. (2014) reported that 10–7 M E2 in MCF-7 cells can down-regulate miR-148a expression which is a HLA-G inhibitor and in resulting HLA-G can increase [29]. In our experiment, we did not detect significant values of HLA-G positive ADSCs in 10–7 M E2 compared to control group. Maybe; it was related to different types of the cells in two tests. Yet, more studies are required to support this hypothesis.
In the other hand, Ing et al. have proved that 50 µg/ml (~20×10–6M) E2 coordinately induces an increase in the number of PgR and ER and enhances the capacity of progesterone in some cells such as oviduct but it may be absent in some organs such as spleen or lung [1819]. We could not detect significant value of HLA-G positive cells in this blend maybe, it be related to E2 concentrations used in our study because E2 concentrations were 10–9 and 10–7 M in our study. Therefore, P4 plus E2 complex did not affect on increasing HLA-G.
In final, it should be pointed out that progesterone and estradiol regulatory effect in the HLA-G expression are dose-dependent. So, it is being seen that progesterone has more strength effect than estradiol in the same culture conditions. Probably, the range of E2 concentration was low or incubation time was short for gaining better result; though ample evidences are required to confirm this hypothesis.
ACKNOWLEDGEMENTS
This study was supported by deputy of chancellor research of Isfahan University of Medical Science.
References
1. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013; 34:747–754. PMID: 23736003.

2. Choi JS, Leem JW, Lee KH, Kim SS, Suh-Kim H, Jung SJ, Kim UJ, Lee BH. Effects of human mesenchymal stem cell transplantation combined with polymer on functional recovery following spinal cord hemisection in rats. Korean J Physiol Pharmacol. 2012; 16:405–411. PMID: 23269903.

3. Alipour R, Sadeghi F, Hashemi-Beni B, Zarkesh-Esfahani SH, Heydari F, Mousavi SB, Adib M, Narimani M, Esmaeili N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int J Prev Med. 2010; 1:164–171. PMID: 21566786.
4. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005; 129:118–129. PMID: 15801964.

5. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8. PMID: 21352202.

6. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–3506. PMID: 17664353.

7. Amodio G, Gregori S. Distinctive immunological functions of HLA-G. Division of regenerative medicine, stem cells and gene therapy. 2nd ed. Milan, Italy: San Raffaele Scientific Institute;2012. 5.
8. Castelli EC, Veiga-Castelli LC, Yaghi L, Moreau P, Donadi EA. Transcriptional and posttranscriptional regulations of the HLA-G Gene. J Immunol Res. 2014; 2014.
9. Yie SM, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006; 21:2538–2544. PMID: 16684846.

10. Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003; 81:199–252. PMID: 14711057.

11. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107:367–372. PMID: 16141348.

12. DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, García L, Abad JL, Trigueros C, Delgado M, Büscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009; 15:2795–2806. PMID: 19231921.
13. Tatara R, Ozaki K, Kikuchi Y, Hatanaka K, Oh I, Meguro A, Matsu H, Sato K, Ozawa K. Mesenchymal stromal cells inhibit Th17 but not regulatory T-cell differentiation. Cytotherapy. 2011; 13:686–694. PMID: 21171824.

14. Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000; 183:682–688. PMID: 10992193.

15. Zhao X, Liu L, Liu D, Fan H, Wang Y, Hu Y, Hou Y. Progesterone enhances immunoregulatory activity of human mesenchymal stem cells via PGE2 and IL-6. Am J Reprod Immunol. 2012; 68:290–300. PMID: 22747921.

16. Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol. 2009; 296:C317–C326. PMID: 18987249.
17. Ragerdi Kashani I, Hedayatpour A, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Mamoudi R, Baazm M. 17β-Estradiol enhances the efficacy of adipose-derived mesenchymal stem cells on remyelination in mouse model of multiple sclerosis. Acta Med Iran. 2012; 50:789–797. PMID: 23456519.
18. Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997; 56:1205–1215. PMID: 9160720.
19. O'Malley BW, Sherman MR, Toft DO. Progesterone "receptors" in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A. 1970; 67:501–508. PMID: 5289005.
20. Ansar MM, Esfandiariy E, Mardani M, Hashemibeni B, Zarkesh-Esfahani SH, Hatef M, Kabiri A. A comparative study of aggrecan synthesis between natural articular chondrocytes and differentiated chondrocytes from adipose derived stem cells in 3D culture. Adv Biomed Res. 2012; 1:24. PMID: 23210083.

21. Cohen-Solal K, Bailly A, Rauch C, Quesne M, Milgrom E. Specific binding of progesterone receptor to progesterone-responsive elements does not require prior dimerization. Eur J Biochem. 1993; 214:189–195. PMID: 7685278.

22. Yie SM, Li LH, Li GM, Xiao R, Librach CL. Progesterone enhances HLA-G gene expression in JEG-3 choriocarcinoma cells and human cytotrophoblasts in vitro. Hum Reprod. 2006; 21:46–51. PMID: 16210391.

23. Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, Fouillard L. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007; 84:231–237. PMID: 17667815.

24. Blanco O, Tirado I, Muñoz-Fernández R, Abadía-Molina AC, García-Pacheco JM, Peña J, Olivares EG. Human decidual stromal cells express HLA-G: Effects of cytokines and decidualization. Hum Reprod. 2008; 23:144–152. PMID: 17951263.

25. Sheshgiri R, Rao V, Tumiati LC, Xiao R, Prodger JL, Badiwala M, Librach C, Delgado DH. Progesterone induces human leukocyte antigen-g expression in vascular endothelial and smooth muscle cells. Circulation. 2008; 118(14 Suppl):S58–S64. PMID: 18824770.

26. Ivanova-Todorova E, Mourdjeva M, Kyurkchiev D, Bochev I, Stoyanova E, Dimitrov R, Timeva T, Yunakova M, Bukarev D, Shterev A, Tivchev P, Kyurkchiev S. HLA-G expression is up-regulated by progesterone in mesenchymal stem cells. Am J Reprod Immunol. 2009; 62:25–33. PMID: 19527229.
27. Mouillot G, Marcou C, Zidi I, Guillard C, Sangrouber D, Carosella ED, Moreau P. Hypoxia modulates HLA-G gene expression in tumor cells. Hum Immunol. 2007; 68:277–285. PMID: 17400064.

28. He X, Dong DD, Yie SM, Yang H, Cao M, Ye SR, Li K, Liu J, Chen J. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol. 2010; 17:1459–1469. PMID: 20052552.

29. Tao S, He H, Chen Q, Yue W. GPER mediated estradiol reduces miR-148a to promote HLA-G expression in breast cancer. Biochem Biophys Res Commun. 2014; 451:74–78. PMID: 25063027.

Fig. 1
Flow cytometry dot plot of HLA-G positive ADSCs in the absence of progesterone and estradiol.
(A) Isotype control. (B) Control group. Shown percentages are the mean values of three independent experiments.
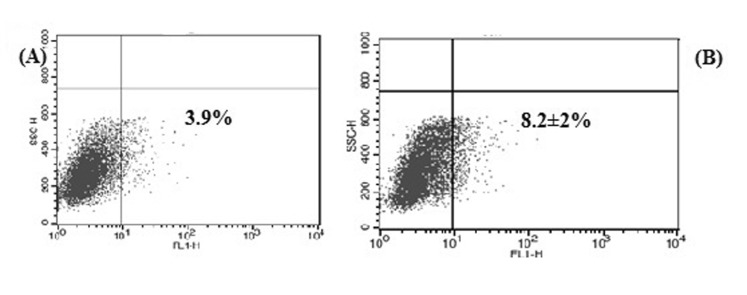
Fig. 2
Flow cytometry dot plot of HLA-G positive ADSCs in the presence of progesterone.
(A) 1×10–5 M P4, (B) 3×10–5 M P4. (C) Statistical analysis of the percentage of HLA-G positive P4-treated cells compared to control cell. Shown percentages are the mean values of three independent experiments. ***<0.001; ns, not significant; c, control cell.
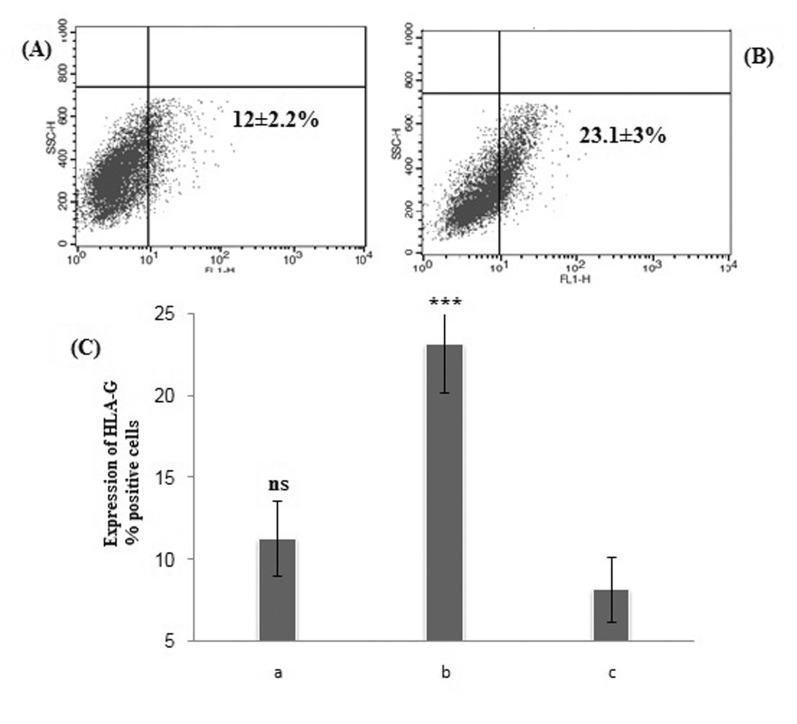
Fig. 3
Flow cytometry dot plot of HLA-G positive ADSCs in the presence of estradiol.
(A) 1×10–9 M E2, (B) 1×10–7 M E2. (C) Statistical analysis of the percentage of HLA-G positive E2-treated cells compared to control cell. Shown percentages are the mean values of three independent experiments (p>0.05). ns, not significant; c, control cell.
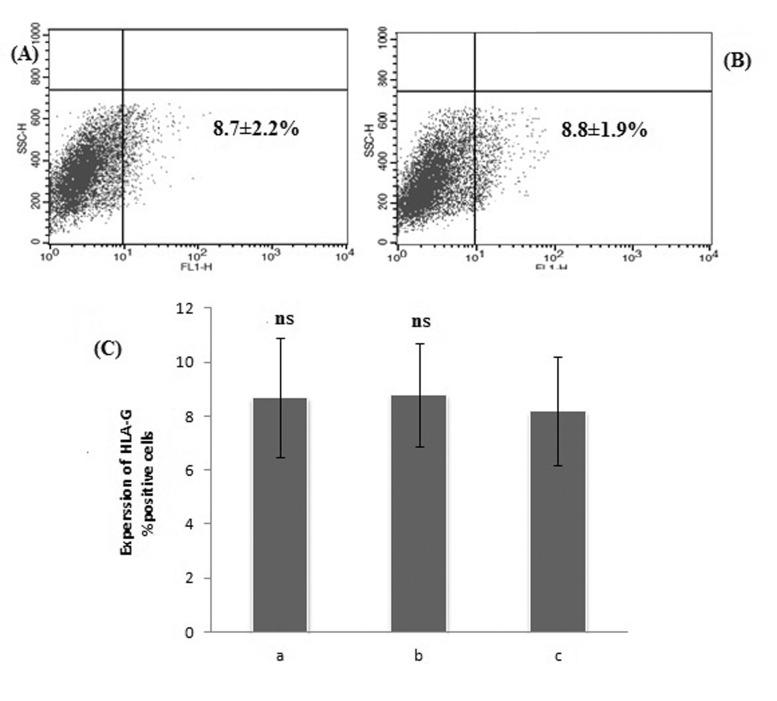
Fig. 4
Flow cytometry dot plot of HLA-G positive ADSCs in the presence of P4 plus E2.
Panels (A) E2: 10–7 M+P4:1×10–5 M, (B) E2: 10–7 M+P4:3×10–5 M, (C) E2:10–9 M+P4:1×10–5 M, (D) E2:10–9 M+ P4:3×10–5 M. (E) Statistical analysis of the percentage of HLA-G positive P4 plus E2-treated cells compared to P4-treated cells as control group. Shown percentages are the mean values of three independent experiments (p>0.05). ns, not significant. C1: 1×10–5 M P4, C2: 3×10–5 M P4.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download