Abstract
Ion channels in carcinoma and their roles in cell proliferation are drawing attention. Intracellular Ca2+ ([Ca2+]i)-dependent signaling affects the fate of cancer cells. Here we investigate the role of Ca2+-activated K+ channel (SK4) in head and neck squamous cell carcinoma cells (HNSCCs) of different cell lines; SNU-1076, OSC-19 and HN5. Treatment with 1 µM ionomycin induced cell death in all the three cell lines. Whole-cell patch clamp study suggested common expressions of Ca2+-activated Cl- channels (Ano-1) and Ca2+-activated nonselective cation channels (CAN). 1-EBIO, an activator of SK4, induced outward K+ current (ISK4) in SNU-1076 and OSC-19. In HN5, ISK4 was not observed or negligible. The 1-EBIO-induced current was abolished by TRAM-34, a selective SK4 blocker. Interestingly, the ionomycin-induced cell death was effectively prevented by 1-EBIO in SNU-1076 and OSC-19, and the rescue effect was annihilated by combined TRAM-34. Consistent with the lower level of ISK4, the rescue by 1-EBIO was least effective in HN5. The results newly demonstrate the role of SK4 in the fate of HNSCCs under the Ca2+ overloaded condition. Pharmacological modulation of SK4 might provide an intriguing novel tool for the anti-cancer strategy in HNSCC.
Head and neck squamous cell carcinoma (HNSCC) is a challenging disease. The cancer itself and its treatments impair the quality of life. In addition to the changes of the physical appearance, it causes deficits in speech, swallowing, taste and olfaction. To preserve the organ and its function, chemotherapy and radiation therapy are preferred to surgical resection in many patients with locally advanced diseases [1]. However, the chemotherapeutic agents are usually unspecific to the cancer cells, causing various complications damaging the normal cells and tissues. The efficacy of the molecular targeted agents for HNSCC is still very limited and the conventional chemotherapeutic agents such as cisplatin are still used. Therefore, further investigation of chemical agents affecting the proliferation and death of HNSCC is still requested.
Ion channels are critical players of physiological functions and pathophysiological processes [2]. Ion channels are activated by variety of physicochemical factors and intracellular second messengers such as Ca2+ ion. The changes in cytosolic Ca2+ ([Ca2+]c) are highly important and influence a number of ion channel activities. The representative Ca2+-activated channels are, 1) two subfamily of Ca2+- activated K+ (KCa) channels (e.g. BKCa and SKCa (SK1 - 4), (2)) Ca2+- activated nonselective cation (CAN) channels (e.g. TRPM4 and 5), and Ca2+-activated Cl- (ClCa) channels (e.g. Ano1/TMEM16A) [34567].
The ClCa current equivalent to functional expression of Ano-1 is well known in squamous epithelial cells such as keratinocytes [89]. In the HNSCC studies, Ano-1 has been suggested to play a role in metastasis and proliferation. Efflux of Cl- is accompanied by water flux and subsequent cell volume changes. Such changes are thought to underlie the migration through narrow intercellular spaces and tumor metastasis. In fact, genomic amplification and protein expression of Ano-1 have been suggested as strong predictors of poor outcome in HNSCC [101112].
Secretory types of epithelial cells express various KCa as well as ClCa channels [1314151617]. However, studies on KCa channels are rare in the squamous epithelial cells [18], and lacking in HNSCCs. The K+ channel activation is generally responsible for hyperpolarized membrane potential. The K+ channel-dependent negative membrane voltage provides electrical driving force for the concomitant transport of other ions along with essential nutrients such as glucose and amino acids. In addition, the level of membrane potential affects cell cycle regulation and survival [19]. In some types of apoptotic conditions, excessive activation of K+ efflux is regarded to be responsible for apoptotic volume decrease due to accompanied Cl- and water efflux [202122].
Sustained increase in [Ca2+]c and subsequent Ca2+ overload in intracellular organelles (e.g. mitochondria) are generally thought to be harmful for cells and would induce cell death depending on the level of [Ca2+]c and on the cell types. In fact, an application of ionophore such as ionomycin has been used as a cell death inducing condition in cancer cells [232425]. Under the sustained increase in [Ca2+]c, ion channels would be also activated. Interestingly, Ano-1 has been reported to play roles in the migration and proliferation of HNSCCs and prostate cancer cells [1026]. However, previous studies have not paid attention to the role of KCa channels of Ca2+-overloaded cancer cells, especially in HNSCCs.
On these backgrounds, we initially investigate the effects of ionomycin on the ion channel currents including ISK4 in HNSCCs. Secondly, the effects of pharmacological activators and inhibitor of the SK4 on the ionomycin-induced cell death are compared between the HNSCC cell lines. The present results demonstrate that pharmacological activation of SK4 effectively rescues HNSCCs from the ionomycin-induced cell death.
We were used three kinds of HNSCC cell lines in this study; SNU-1076, OSC-19, and HN5. SNU-1076 cells are originated from laryngeal cancer. OSC-19 and HN5 are originated from oral squamous cell carcinoma. SNU-1076, OSC-19, HN5 cells were maintained in RPMI-1640 media with 10% (v/v) fetal bovine serum (GIBCO), 1% antibiotic/antimycotic (GIBCO) and 0.1% Gentamicin Reagent solution (GIBCO) at 37℃ in a humidified, 5% CO2, 95% air atmosphere and routinely sub-cultured using trypsin-EDTA. For proliferation assay, cells were seeded in culture plates and incubated for the specific time at 37℃ prior to treatment with specific drugs for the indicated time. After treatment, Cell Counting Kit-8 (Dojindo Lab., Tokyo, Japan) was used to measure cell proliferation according to the manufacturer's instructions.
Whole-cell patch clamp experiments were performed on HN5, OSC-19, and SNU-1076 cells at room temperature (22~25℃). Ionic currents and membrane potential were recorded in voltage-clamp and current-clamp mode, respectively, using Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster, CA, USA). Recorded traces were digitized, sampled at 10 kHz, and low-pass filtered at 2 kHz using Digidata 1440A converter (Axon Instrument). Sampled data were stored on a computer using Clampex of pClamp 10.1 software (Axon Instrument). The patch-clamp data was analyzed by using Clampfit 10.3 and Origin 7.0 software (Microcal Inc., Northampton, MA, USA). Glass patch pipettes (World Precision Instruments, Sarasota, FL, USA) were pulled with PP-830 (Narishige, Tokyo, Japan) to make tip resistance 2.5~3 MΩ when filled with KCl or CsCl pipette solution.
For the whole-cell patch clamp experiment, low Ca2+-buffered KCl pipette solution contained (in mM): 140 KCl, 10 HEPES, 5 NaCl, 3 Mg-ATP, 0.1 EGTA, 1 MgCl2 and was adjusted to pH 7.2 with KOH. Ca2+-clamped KCl pipette solution contained (in mM) 140 KCl, 1 MgCl2, 3 Mg-ATP, 10 HEPES, 5 EGTA, 5 NaCl, and 300 nM free Ca2+ calculated using the free WebMax software (www.stanford.edu/~cpatton/webmaxcS), and was adjusted to pH 7.25 with KOH. Ca2+-clamped CsCl pipette solution contained (in mM): 140 CsCl, 1 MgCl2, 3 Mg-ATP, 10 HEPES, 5 EGTA, 5 NaCl, and 600 nM free Ca2+calculated using the free WebMax software and was adjusted to pH 7.25 with CsOH. Normal Tyrode's (NT) bath solution contained (in mM): 145 NaCl, 3.6 KCl, 1 MgCl2, 1.3 CaCl2, 10 HEPES, 5 glucose, 10 sucrose and was adjusted to pH 7.4 with NaOH. NMDG-Cl bath solution contained: 150 NMDG-Cl, 10 HEPES, 10 glucose, 1 MgCl2, 1.3 CaCl2 and was adjusted to pH 7.4 with NMDG-OH. Ionomycin, 1-ethyl-2-benzimidazolinone (1-EBIO) and 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) were purchased from Sigma-Aldrich. T16Ainh-A01, a specific inhibitor of Ano-1, was purchased from TOCRIS Biosciences. The chemicals were initially dissolved in dimethyl sulfoxide (DMSO), and were diluted to the bath solution for experiments. The amount of DMSO in the bath solution was kept below 0.1%.
Total RNA was extracted from cancer cells using the Trizol (Invitrogen, CA, USA), and 2 µg of total RNA was reverse transcribed into first-strand cDNA. Reverse transcription was performed with RT premix (Intron Bio, Korea) according to the manufacturer's instructions. The cDNA was amplified by 35 cycles of PCR with PCR PreMix (Intron, Korea) in a C1000 Thermal Cycler (bio-rad, CA, USA). The SK1-4 primers were obtained from Bioneer (Bioneer, Korea). The expected product sizes from the customized primer sets were 155, 162, 150, and 154 bp for SK1 (product No. P243222), SK2 (P243234), SK3 (P114469) and SK4 (P249299), respectively. GAPDH was used as control. RT-PCR products were resolved by 1.2% agarose gel electrophoresis and visualized with ethidium bromide staining.
Data are presented as the mean±standard deviation (SD) of at least triplicates, or as a representative of 3 separate experiments. Significance was determined between treated and untreated groups by two-sided Student's t-test. p values <0.05 were considered statistically significant.
Firstly, ionomycin-induced changes in membrane conductance were investigated in the three cell lines; SNU-1076, OSC-19, and HN5. Low Ca2+-buffered KCl pipette solution (0.1 mM EGTA) was used to allow [Ca2+]i increase in response to the ionomycin treatment. Ramp-like voltage change from -100 to 60 mV was repetitively applied at every 10 s. During interpulse period, the cell membrane voltage was held at -60 mV (Fig. 1A, C, E). Before ionomycin application, membrane conductance was commonly low in the three cell lines; small amplitudes at both negative and positive voltages (Fig. 1B, D, F; black I/V curves labelled (1) in the right panels). Application of ionomycin gradually increased the membrane conductance (i.e. the slope of I/V curve) in the three cell lines. However, the dominant type of ionic conductance at the earlier period appeared different between the cell lines. In SNU-1076, outward K+ current was initially activated as indicated from the upward deflection of basal current trace at the holding voltage of -60 mV (Fig. 1A, red I/V curve labelled (2)). Consistently, the I/V curve showed outward currents that reversed its direction at around -80 mV, close to the K+ equilibrium potential (Fig. 1B, I/V curve in red). The weakly inwardly rectifying shape of I/V curve was consistent with the property of SK4. In the later phase of ionomycin application, however, the outward current disappeared with concomitant activation of inward current. Also the I/V curve at the later period (ca. 4~5 min of ionomycin treatment) showed linear shape with reversal potential close to 0 mV (Fig. 1B, blue I/V curve labelled (3)). Different from SNU-1076, OSC-19 and HN5 cells did not show discernible SK4-like current but only showed inward currents at the negative clamp voltage (Fig. 1C, E). The I/V curves showed linear shapes with reversal potentials close to 0 mV (Fig. 1D, F, red I/V curves).
The I/V curves of ionomycin-induced currents in their steadystate with 0 mV of reversal potential suggested that either ClCa (Ano-1) or CAN channels were activated. To evaluate the relative contribution of ClCa and CAN channels in HNSCC, CsCl pipette solution with fixed level of high [Ca2+]c (600 nM) was adopted in the next whole-cell patch clamp experiment (Fig. 2). K+ current was excluded by replacing the intracellular K+ with Cs+ that are impermeable to K+ channels. The membrane current increased slowly after making the whole-cell configuration. After confirming a steady-state (I/V curves in black, Fig. 2A~C), 10 µM T16A, a selective inhibitor of Ano1, commonly decreased the conductance in the three cell lines (I/V curves in red, Fig. 2A~C). Then, in the presence of T16A, all the extracellular Na+ was replaced by NMDG+, a large organic cation impermeable to cation channels. On the NMDG+ replacement, additional decrease of inward current was observed at the negative membrane voltages (I/V curves in green, Fig. 2A~C). The current density normalized to the steady-state control current (%) showed that about half of the current was inhibited by T16A in all three cell types (Fig. 2D). In OSC-19, most of the remaining inward current was abolished by replacing extracellular Na+ with NMDG+ (Fig. 2B, D). In SNU-1076 and HN5, however, 10~20% of the conductance was remained even after the removal of Na+. The nature of T16A- and NMDG+-resistant conductance was not further investigated here.
To investigate the role of Ano-1 and SK4 in the Ca2+-overloaded HNSCCs, viability was assessed in the ionomycin-treated cells with T16A (Ano-1 inhibitor) or TRAM-34 (SK4 inhibitor). The number of live cells was counted at 48 h after 1 µM ionomycin treatment, and the numbers were commonly decreased in all the three cell lines. However, neither T16A nor TRAM-34 changed the tendency of ionomycin-induced suppression of proliferation in all the three cell lines (Fig. 3A). Then we tested the effect of 1-EBIO, an activator of SK channels, on the ionomycin-treated HNSCCs. Interestingly, the co-treatment of 1-EBIO with ionomycin effectively recovered the live cell numbers close to the control levels in SNU-1076 and OSC-19. The rescue effect of 1-EBIO was observed already from the relatively low range of concentration (3 and 10 µM), and was completely reversed by the SK4 channel specific inhibitor TRAM-34 (Fig. 3B). However, 1-EBIO rescued the ionomycin-treated HN5 cells only to a small extent (Fig. 3B). The treatment with 1-EBIO or TRAM-34 without ionomycin did not significantly affect the viability of HNSCCs. Representative data for SNU-1076 is show in Fig. 3C.
The rescue effect of 1-EBIO on OSC-19 was unexpected since ionomycin did not directly induce noticeable ISK4 in OSC-19 (Fig. 1C, D). Therefore we were attempted to evaluate whether 1-EBIO actually induces ISK4 in OSC-19. Here we used KCl pipette solution containing 300 nM of free Ca2+ activity. With the moderate level of [Ca2+]c alone, i.e. 300 nM, Ca2+-activated ion channel current was not significantly observed. However, the treatment with 1-EBIO induced outward K+ currents with weakly inward rectifying property in the three cell lines (Fig. 4). Interestingly, the current density of 1-EBIO-induced ISK4 was largest in OSC-19 and smallest in HN5 (Fig. 4D). Then we performed RT-PCR analysis for SK1-4 in the three cell lines, as was detected from the pharmacological assay of membrane current (Fig. 4D), SK4 transcript was conformed in SNU-1076 and OSC-19 while not in HN5 (Fig. 4E). However, unexpectedly, all the three cell lines showed positive signal for the mRNA of SK1. Since the 1-EBIO, activator of SK channels-activated current was negligible in HN5, and the K+ current induced by 1-EBIO completely inhibited by the SK4 channel specific inhibitor TRAM-34 in SNU-1076 and OSC-19 cell lines, we interpreted that the functional SK1 channel proteins not expressed in the three cell lines (Fig. 4E).
Then we investigated whether 1-EBIO also affect the K+ conductance of ionomycin-treated cells with unclamped [Ca2+]i conditions, i.e. 0.1 mM EGTA in KCl pipette solution. As shown in Fig. 1, application of 1 µM ionomycin induced outward K+ current in SNU-1076 whereas inward currents in OSC-19 and HN5. Interestingly, addition of 50 µM 1-EBIO induced large outward current in both OSC-19 and SNU-1076. Subsequent application of TRAM-34 effectively abolished the 1-EBIO-induced K+ currents (Fig. 5A~D). In HN5 cells, however, application of 1-EBIO still induced only minute increase of membrane conductance (Fig. 5E~F).
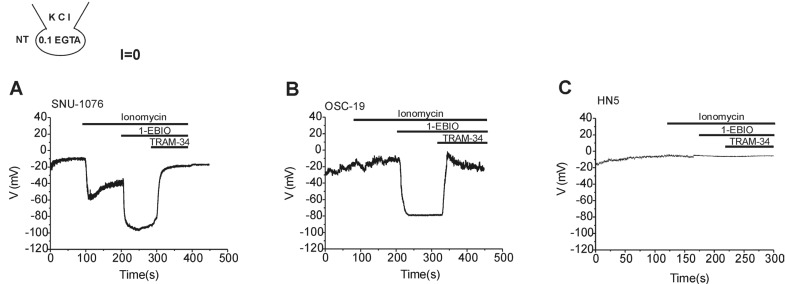
The activation of ISK4 by 1-EBIO implied membrane hyperpolarization as suspected from the negative shift of reversal potentials (Fig. 5B and D). To confirm the actual effects on membrane potential, zero-current clamp recording was performed. Ionomycin, 1-EBIO and TRAM-34 were serially applied to HNSCCs with 0.1 mM EGTA/145 mM KCl in the pipette solution. All the three cell lines showed depolarized membrane potential between -15 and 0 mV at the steady-state of whole-cell configuration. In SNU-1076, ionomycin induced initial negative shift of membrane potential, which was further hyperpolarized by 1-EBIO (Fig. 6A). In OSC-19, ionomycin induced a slight depolarization, which was reversed to strong hyperpolarization by 1-EBIO (Fig. 6B). The final application of TRAM-34 depolarized the membrane potential in both SNU-1076 and OSC-19. In HN5, however, neither ionomycin nor 1-EBIO induced consistent changes in the membrane potential (Fig. 6C).
Our present study newly demonstrates that HNSCCs differentially express functional SK4 channels as revealed by the amplitudes of 1-EBIO-induced ISK4. The pharmacological activation of ISK4 seemed to rescues the HNSCCs from ionomycin-induced cell death. The abolishment of 1-EBIO effects by TRAM-34 is also the evidence indicating specific role of SK4. The activation of SK4, i.e. K+ efflux and membrane hyperpolarization appear to be the critical conditions for the rescue effect. In fact, the rescue effect was least significant in HN5 cells where the current density of KCa3.1 was smaller than the other cell lines.
In the resting HNSCCs without raised [Ca2+]c, little background ionic conductance was observed; the slopes of I/V curves were very shallow in all the three cell lines tested. In contrast, experimental measures increasing [Ca2+]c consistently activated multiple types of ion channels permeating K+ (SK4), Cl- (ClCa) and Na+ (CAN) with differential contributions depending on the cell types. Molecular identity of the ClCa has been recently discovered as Ano-1/TMEM16A, and found in HNSCCs [1027]. The significant decrease of Ca2+-activated inward current by T16A indicates the expression of Ano1 in the three lines of HNSCCs investigated here. In addition to Ano-1, the incomplete inhibition by T16A and further decrease by replacing Na+ with NMDG+ indicate that CAN channels are expressed in HNSCCs (Fig. 2). In the present study, the precise molecular identity of CAN was not investigated. The molecular identity of CAN is generally ascribed to TRPM4 and TRPM5, members of TRP channel superfamily [56].
The activation of ClCa and CAN would commonly induce depolarization of HNSCC close to their Nernst equilibrium voltages (Veq) of Cl- or monovalent cations in the present experimental conditions. Therefore large increase in [Ca2+]c would inevitably set the membrane potential of HNSCCs close to 0 mV. Since the HNSCCs do not show background K+ conductance, their resting membrane potentials are already depolarized. The lack of voltage changes by ionomycin alone in OSC-19 and HN5 seem to be due to the already depolarized resting membrane potentials of HNSCCs (Fig. 6). In other words, the application of ionomycin would further stabilize the depolarized states. Even in 1076 cells where the activation by SK4 by ionomycin was evident, the application of ionomycin induces only a moderate hyperpolarization (Fig. 6A).
A curious finding of our present study was the requirement of 1-EBIO for the activation of SK4 in OSC-19. Although the final amplitude of 1-EBIO-induced ISK4 was comparable or even larger in OSC-19 than SNU-1076, the application of ionomycin did not effectively activate ISK4 in OSC-19. Such results might imply that the activation of SK4 might require, in addition to [Ca2+]c increase, unknown factors or molecular components that are selectively modulated by 1-EBIO in HNSCCs.
The effects of 1-EBIO on the cell viability of ionomycin-treated HNSCCs seem to be associated with the level of ISK4 because TRAM-34 effectively reversed the rescue effect by 1-EBIO (Fig. 3). Thus one can suggested that the full activation of SK4 and strong hyperpolarization are critical for the effects of 1-EBIO. What is the precise target or mechanism of SK4 activation in the cell proliferation of HNSCCs? Unfortunately, we do not have concrete answers to that question yet but could only provide general inquiry of previous studies.
The importance of K+ channel activity in cell cycle regulation and proliferation/death has been investigated in various aspects and the responses are widely variable from proliferation to cell death [222829]. In the studies of apoptosis, it is proposed that the excessive activation of K+ channel and hyperpolarization would induce continuous efflux of Cl- as well as K+, which is accompanied by the loss of cytoplasmic water and cell volume, causing apoptotic volume decrease (AVD) [2230]. In this respect, the excessive activation of SK4 in line with Ano-1 may also induce cell volume decrease in SNU-1076 and OSC-19. However, the outcome of the cell viability was opposite to the K+-channel dependent AVD suggested by Bortner's group [22]. Thus the final effects of K+ efflux on cell proliferation seem to be variable or could even show opposite results depending on the con-text of intracellular signals and the cell types.
Partly consistent with our present results, the previous studies of breast, prostate and pancreatic cancer cells commonly report the inhibition of cell proliferation by SK4 inhibitor [3132]. In another study, the proliferation of human prostate cancer cell lines (LNCaP and PC-3) was increased by 1-EBIO, and this effect was again reversed by SK4 inhibitors [33]. Regarding to the mechanism, it has been proposed that the hyperpolarization and augmented Ca2+ influx promotes the transition of cell cycle phases and thereby proliferation [3234]. Since the treatment of HNSCCs with ionomycin alone would induce significant increase in [Ca2+]c, the theory of augmented Ca2+ influx by ISK4- induced hyperpolarization seems unlikely for the rescue effect of 1-EBIO. In fact, a measurement of intracellular [Ca2+]c with fura-2 fluorometry consistently showed large increase in [Ca2+]c by ionomycin alone, that was not significantly augmented by 1-EBIO in SNU-1076 and OSC-19 (n=5, data not shown). Recently, Millership et al. proposed that SK4 may directly activate ERK1/2 and JNK signaling pathways in a voltage-independent way, which promotes proliferation [35]. The putative non-electrical role of SK4 directly interacting with the intracellular signaling molecules might have to be also considered in HNSCCs. Apart from the Ca2+-activated K+ channels, background type K+ channels such as TREK-2 could also regulate the cancer cell proliferation [36].
In summary, this study firstly demonstrates the expression of SK4 and functional activation by 1-EBIO in HNSCC. Although the precise mechanisms are not known yet, the activation of ISK4 and membrane hyperpolarization induced by 1-EBIO effectively rescue the ionomycin-induced cell death in the HNSCCs expressing SK4. Pharmacological modulation of SK4 might provide a hint for the development of more effective anti-cancer agent or better strategy for cancer treatment.
ACKNOWLEDGEMENT
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (Grants NRF 2011-0017370 and NRF 2012-0000809). Also, this study was supported by a grant from Seoul National University Hospital (2015).
Notes
Author contributions: M.Z.Y., S.W.P., T.W.K. and K.S.K. performed the electrophysiological and cell-based assay experiments. H.Y.Y., J.H.H., J.H.L. contributed critical discussion and design of experiments. M.H.S. and S.J.K. supervised and coordinated the study. M.Z.Y., S.W.P, and S.J.K. wrote the manuscript.
References
1. Lim Y, Keam B, Koh Y, Kim TM, Lee SH, Hah JH, Kwon TK, Kim DW, Wu HG, Sung MW, Heo DS, Kim KH. Clinical outcomes of radiation-based locoregional therapy in locally advanced head and neck squamous cell carcinoma patients not responding to induction chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:55–60. PMID: 23570665.

3. Ge L, Hoa NT, Wilson Z, Arismendi-Morillo G, Kong XT, Tajhya RB, Beeton C, Jadus MR. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int Immunopharmacol. 2014; 22:427–443. PMID: 25027630.

4. Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004; 5:758–770. PMID: 15378036.
5. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002; 109:397–407. PMID: 12015988.
6. Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003; 100:15166–15171. PMID: 14634208.
7. Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev. 2014; 94:419–459. PMID: 24692353.

8. Zholos A, Beck B, Sydorenko V, Lemonnier L, Bordat P, Prevarskaya N, Skryma R. Ca2+- and volume-sensitive chloride currents are differentially regulated by agonists and store- operated Ca2+ entry. J Gen Physiol. 2005; 125:197–211. PMID: 15657298.
9. Park SJ, Choi WW, Kwon OS, Chung JH, Eun HC, Earm YE, Kim SJ. Acidic pH-activated Cl- current and intracellular Ca2+ response in human keratinocytes. Korean J Physiol Pharmacol. 2008; 12:177–183. PMID: 19967053.
10. Ruiz C, Martins JR, Rudin F, Schneider S, Dietsche T, Fischer CA, Tornillo L, Terracciano LM, Schreiber R, Bubendorf L, Kunzelmann K. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS One. 2012; 7:e43265. PMID: 22912841.

11. Wanitchakool P, Wolf L, Koehl GE, Sirianant L, Schreiber R, Kulkarni S, Duvvuri U, Kunzelmann K. Role of anoctamins in cancer and apoptosis. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130096. PMID: 24493744.

12. Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012; 72:3270–3281. PMID: 22564524.

13. Girault A, Brochiero E. Evidence of K+ channel function in epithelial cell migration, proliferation, and repair. Am J Physiol Cell Physiol. 2014; 306:C307–C319. PMID: 24196531.
14. Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011; 286:19830–19839. PMID: 21454692.
15. Takahata T, Hayashi M, Ishikawa T. SK4/IK1-like channels mediate TEA-insensitive, Ca2+-activated K+ currents in bovine parotid acinar cells. Am J Physiol Cell Physiol. 2003; 284:C127–C144. PMID: 12388063.
16. Hayashi M, Wang J, Hede SE, Novak I. An intermediate-conductance Ca2+-activated K+ channel is important for secretion in pancreatic duct cells. Am J Physiol Cell Physiol. 2012; 303:C151–C159. PMID: 22555847.
17. Jang Y, Oh U. Anoctamin 1 in secretory epithelia. Cell Calcium. 2014; 55:355–361. PMID: 24636668.

18. Shieh DB, Yang SR, Shi XY, Wu YN, Wu SN. Properties of BK(Ca) channels in oral keratinocytes. J Dent Res. 2005; 84:468–473. PMID: 15840785.

19. Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009; 8:3527–3536. PMID: 19823012.

20. Pardo LA, Stuhmer W. The roles of K+ channels in cancer. Nat Rev Cancer. 2014; 14:39–48. PMID: 24336491.
21. Ernest NJ, Habela CW, Sontheimer H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J Cell Sci. 2008; 121:290–297. PMID: 18198188.

22. Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130104. PMID: 24493752.

23. Miyake H, Hara I, Yamanaka K, Arakawa S, Kamidono S. Calcium ionophore, ionomycin inhibits growth of human bladder cancer cells both in vitro and in vivo with alteration of Bcl-2 and Bax expression levels. J Urol. 1999; 162:916–921. PMID: 10458408.

24. Han S, Tie X, Meng L, Wang Y, Wu A. PMA and ionomycin induce glioblastoma cell death: activation-induced cell-death-like phenomena occur in glioma cells. PLoS One. 2013; 8:e76717. PMID: 24130787.

25. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003; 4:552–565. PMID: 12838338.

26. Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca2+- activated Cl- channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012; 326:41–51. PMID: 22820160.
27. Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa L, Robe A, Roux M, Abecassis J, de Reynies A, Wasylyk B. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br J Cancer. 2010; 103:715–726. PMID: 20664600.

28. Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130094. PMID: 24493742.

29. Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014; 206:151–162. PMID: 25049269.

30. McFerrin MB, Turner KL, Cuddapah VA, Sontheimer H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol. 2012; 303:C1070–C1078. PMID: 22992678.

31. Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004; 65:630–638. PMID: 14978241.
32. Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004; 287:C125–C134. PMID: 14985237.
33. Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003; 471:157–164. PMID: 12826234.
34. Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackiere F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009; 28:1792–1806. PMID: 19270724.
35. Millership JE, Devor DC, Hamilton KL, Balut CM, Bruce JI, Fearon IM. Calcium-activated K+ channels increase cell proliferation independent of K+ conductance. Am J Physiol Cell Physiol. 2011; 300:C792–C802. PMID: 21123738.
36. Park KS, Han MH, Jang HK, Kim KA, Cha EJ, Kim WJ, Choi YH, Kim Y. The TREK2 channel is involved in the proliferation of 253J cell, a human bladder carcinoma cell. Korean J Physiol Pharmacol. 2013; 17:511–516. PMID: 24381500.

Fig. 1
Ionomycin-induced membranen currents in HNSCCs.
(A, C, E) Representative original current traces in response to repetitive ramp-like pulses (from -100 to 60 mV, holding voltage, -60 mV) in whole-cell voltage clamped cells. The type of cell line is indicated above each trace. KCl pipette solution with 0.1 mM EGTA was commonly used. The numbers in parenthesis indicate the corresponding current to voltage relations (I/V curves) depicted in the right panels (B, D, F). After achieving the whole-cell configuration, the steady-state conductance was very small; black I/V curves that are almost indistinguishable from the horizontal axis. On applying 1 µM ionomycin, membrane conductance increased gradually, and the addition of 5 µM TRAM-34 inhibited outward currents in SNU-1076 (A, B). In contrast, the ionomycin-treated OSC-19 (time break, 1 min) and HN5 showed negligible inhibition by TRAM-34 (C, D).
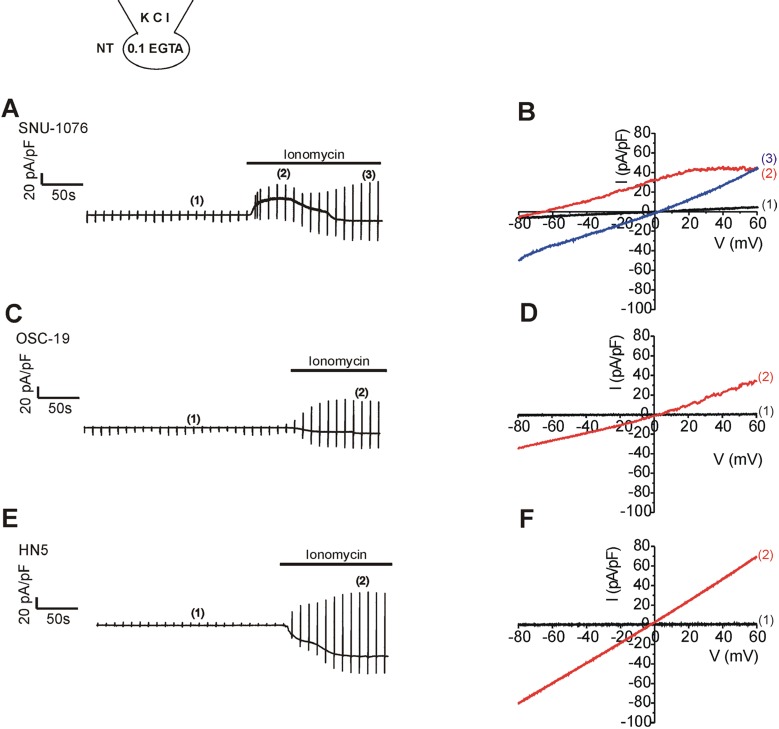
Fig. 2
Ca2+-activated Cl- current and Ca2+-activated nonselective cationic current in HNSCCs.
(A~C) Representative I/V curves obtained by ramplike pulses used CsCl pipette solution with 600 nM free Ca2+ activity in the three types of cell lines. Steady-state Ca2+-activated conductance (black I/V curves in each panel) was decreased by about half after applying 10 M T16A, a selective inhibitor of Ano-1 (I/V curves in red). The remained conductance, especially the inward current, was further decreased by replacing extracellular Na+ with NMDG+ (T16A+NMDG, I/V curves in green). (D) Summary of normalized inward current densities at -60 mV. The inward current density (pA/pF) was normalized (%) to the control steady-state in each cell. The remained current after T16A (red) and Na+ removal (green) are averaged. (E) Summary Ano-1 current densities (pA/pF) at -60 mV in HNSCCs. Ano-1 current was obtained by subtracting T16A-resistent current from control in each cell, and the plotted dots indicate data from individual cell.
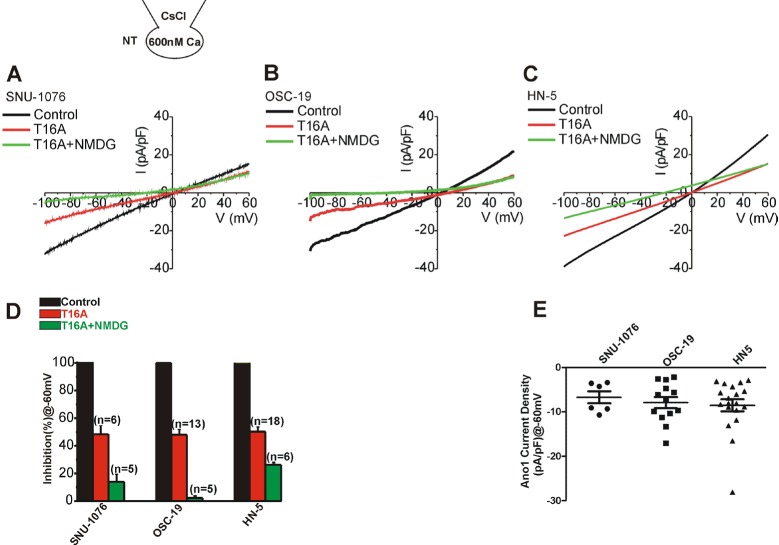
Fig. 3
Ionomycin-induced cell death and rescue effects of 1-EBIO in HNSCCs.
(A) Cells from each cell line were divided into four groups; non-treated (control), treated with 1 µM ionomycin, ionomycin +0.1 µM T16A and ionomycin +10 µM TRAM-34. Cells were subjected to proliferation assay at 48 h and normalized to the control (cell viability). Summary of MTT experiments are displayed. p values were based on comparison with control (*p<0.001). (B) Cells from each cell line were divided into five groups; non-treated (control), treated with 1 µM ionomycin, ionomycin +3 µM 1-EBIO, ionomycin + 10 µM 1-EBIO, and ionomycin/3 µM 1-EBIO +5 µM TRAM-34. Cell numbers at 48 h were counted and normalized to the control (n=6). p values were based on comparison with ionomycin-treated group (*p<0.001) and ionomycin plus 1-EBIO (3 µM)-treated group (#p<0.005). (C) Effects of various concentrations of 1-EBIO or TRAM-34 on the cell viability of SNU-1076 without ionomycin treatment at 48 h.
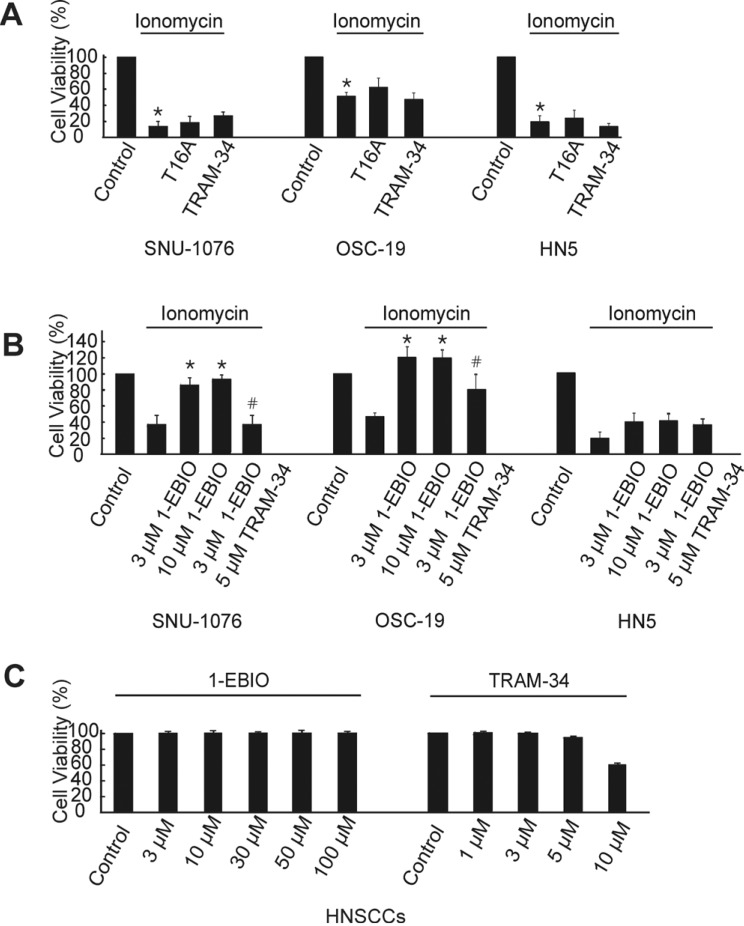
Fig. 4
Effects of 1-EBIO on the membrane conductance of HNSCCs with clamped [Ca2+]i.
(A~C) Representative current traces in response to ramp-like pulses (from -100 to 60 mV, holding voltage, -60 mV) and to the application of 50 µM 1-EBIO in HNSCCs. The Ca2+ activity of KCl pipette solution was equilibrated to 300 nM with 5 mM EGTA. (D) Summary of I/V curves of the 1-EBIO-induced currents in each cell line (mean±SEM, n=7, respectively). (E) Identification of SK mRNA in HNSCC cancer cells. mRNA was detected for SK1 and SK4 by RT-PCR.
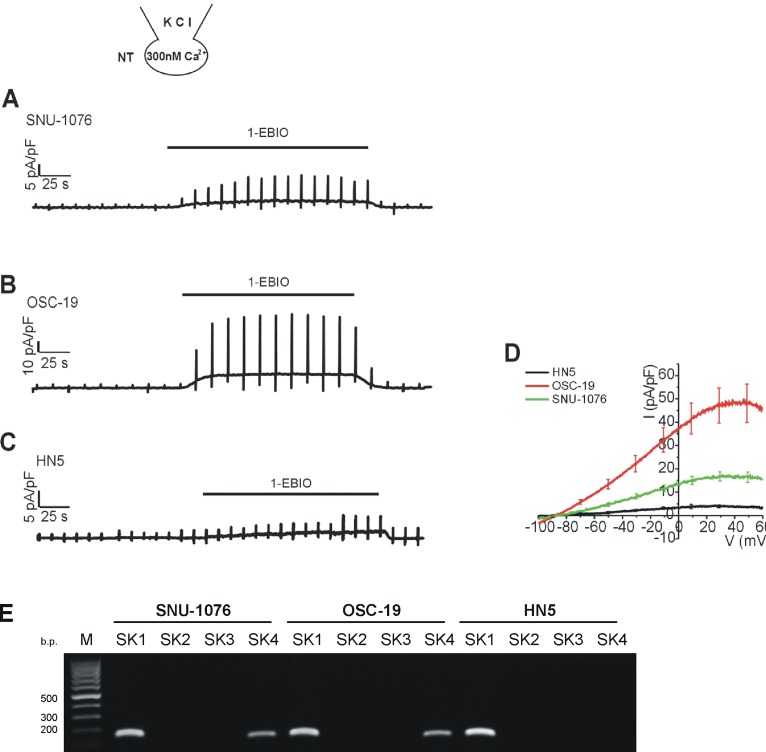
Fig. 5
. Effects of 1-EBIO on the membrane conductance of HNSCCs pretreated with ionomycin under Ca2+-unclamped condition.
Left panels (A, C, E) representative current traces of HNSCCs in response to the depolarizing ramp pulses. 1 µM ionomycin, 50 µM 1-EBIO, and 5 µM TRAM-34 were applied as indicated above each trace. Right panels (B, D, F); I/V curve obtained by the ramp pulses in each cell line of HNSCCs as indicated at the corresponding left panels. The specific timing of I/V curve is indicated by the number in parenthesis. Note the weakly inward rectifying outward currents induced by 1-EBIO in SNU-1076 and OSC-19.
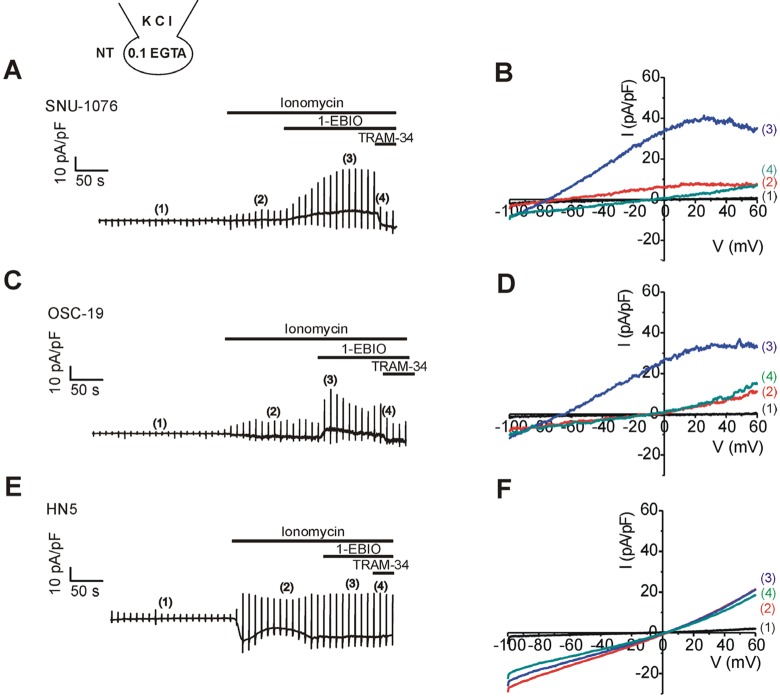
Fig. 6
Membrane voltage changes in response to ionomycin, 1-EBIO and TRAM-34 in HNSCCs.
Traces of membrane potential in HNSCCs obtained under zero-current clamp condition with 0.1 mM EGTA KCl pipette solution. 1 µM ionomycin, 50 µM 1-EBIO, and 5 µM TRAM-34 were applied as indicated in each panel. Representative results from more than five cells from each cell line are shown. Ionomycin alone hyperpolarized the membrane potential in SNU-1076 (A) while the combined application of 1-EBIO induces strong hyperpolarization in both SNU-1076 and OSC-19 (A, B). Final addition of TRAM-34 depolarized the membrane potential in SNU-1076 and OSC-19 (A, B). Neither ionomycin nor 1-EBIO induced significant change in the membrane potential of HN5 (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download