Abstract
The objective of this study was to externally validate a new dosing scheme for busulfan. Thirty-seven adult patients who received busulfan as conditioning therapy for hematopoietic stem cell transplantation (HCT) participated in this prospective study. Patients were randomized to receive intravenous busulfan, either as the conventional dosage (3.2 mg/kg daily) or according to the new dosing scheme based on their actual body weight (ABW) (23×ABW0.5 mg daily) targeting an area under the concentration-time curve (AUC) of 5924 µM·min. Pharmacokinetic profiles were collected using a limited sampling strategy by randomly selecting 2 time points at 3.5, 5, 6, 7 or 22 hours after starting busulfan administration. Using an established population pharmacokinetic model with NONMEM software, busulfan concentrations at the available blood sampling times were predicted from dosage history and demographic data. The predicted and measured concentrations were compared by a visual predictive check (VPC). Maximum a posteriori Bayesian estimators were estimated to calculate the predicted AUC (AUCPRED). The accuracy and precision of the AUCPRED values were assessed by calculating the mean prediction error (MPE) and root mean squared prediction error (RMSE), and compared with the target AUC of 5924 µM·min. VPC showed that most data fell within the 95% prediction interval. MPE and RMSE of AUCPRED were -5.8% and 20.6%, respectively, in the conventional dosing group and −2.1% and 14.0%, respectively, in the new dosing scheme group. These fi ndings demonstrated the validity of a new dosing scheme for daily intravenous busulfan used as conditioning therapy for HCT.
Hematopoietic stem cell transplantation (HCT) is an important therapy for nonmalignant and malignant hematologic diseases. Busulfan is an alkylating agent widely used in combination with other cytotoxic drugs as a myeloablative conditioning regimen for HCT [1234]. Busulfan has a relatively narrow therapeutic window [56]. A large variability in the bioavailability of oral busulfan has been previously reported [7] and, can lead to toxicity, including hepatic veno-occlusive disease [89] as well as graft rejection or suboptimal antitumor activity [10].
To avoid the inherent problems of an oral formulation and improve dosing accuracy, an intravenous (i.v.) formulation of busulfan was developed, which provides a more predictable pharmacokinetic profile and therapeutic efficacy than oral busulfan administration [1112]. A once-daily i.v. busulfan dosing regimen is possible and would be much more convenient and tolerable for both patients and caregivers than a 4 times daily dosage. In addition, several studies have suggested that the once-daily regimen is equally safe and effective, as compared with the 4 times daily administration [131415]. The area under the concentration-time curve (AUC) of busulfan correlates with the clinical outcomes [1617]. A population analysis has previously been applied to investigate the pharmacokinetic characteristics of i.v. busulfan, and a final model for clearance based only on the actual body weight (ABW) of the patient has been proposed [18].
While internal validation is based on basic or more advanced methods such as data splitting and/or resampling techniques [19], external validation is known to be the most stringent method for the evaluation of the predictive ability of a developed model; this approach requires the availability of an external study [202122]. However, external validation studies are relatively rare and have only been reported for 24 of 360 population pharmacokinetic models (6.7%) and for 9 of 118 pharmacodynamic models (7.6%) [22]. There is limited information available currently in relation to the external validation of the population pharmacokinetic model for busulfan in adult patients.
The objective of this study was to evaluate a new, simpler dosing scheme based on ABW for once daily i.v. busulfan (Busulfex®, Orphan Medical, Minnetonka, MN, USA) as conditioning therapy for HCT based on population approaches and using the external validation method. A limited sampling strategy was applied to minimize the number of blood samples required from each patient.
This study was approved by the institutional review board (IRB) of the Asan Medical Center. Informed consent was confirmed by the IRB. Adult patients who received i.v. busulfan on the first day of conditioning therapy for HCT were eligible for study participation. No other chemotherapeutic drugs were allowed on that day. A Karnofsky performance score of 70 or higher and adequate cardiac, hepatic, and renal functions were required for study inclusion. Patients were randomly assigned to 1 of 2 types of dosage regimens: the once daily i.v. conventional dosing scheme (reference arm) or the population model based dosing scheme (test arm). We employed a block randomization method including stratification according to the conditioning regimen (busulfan-cyclophosphamide [BuCy] versus busulfan-fludarabine [BuFlu] versus busulfan-fludarabine-antithymocyte globulin [BuFluATG]).
For the BuCy regimen, i.v. busulfan was administered on days –7 to –4 and cyclophosphamide (60 mg/kg/day) on days –3 and –2. The time between the last dose of busulfan and the first dose of cyclophosphamide was 27 hours. For the BuFlu regimen, we administered i.v. busulfan for 4 days (days –7 to –4) and fludarabine (30 mg/kg) for 5 days (days –6 to –2). For the BuFluATG regimen, we administered i.v. busulfan for 2 days (days –7 and –6), fludarabine (30 mg/kg) for 6 days (days –7 to –2), and antithymocyte globulin. Patients received 1 of 2 types of antithymocyte globulin, according to availability in Korea. Specifically, rabbit antithymocyte globulin (Thymoglobulin®, IMTIX-SANGSTAT, Lyon, France) given as 1.5 mg/kg/day on days –4 to –2 with a matched sibling donor; 3.0 mg/kg/day on days –4 to –2 with an unrelated donor; and 3.0 mg/kg/day on days –4 to –1 with a haplo-identical familial donor; or horse antithymocyte globulin (Lymphoglobulin®, IMTIX-SANGSTAT, Lyon, France) given as 7.5 mg/kg/day on days –4 to –2 with a matched sibling donor; 15.0 mg/kg/day on days –4 to –2 with an unrelated donor; and 15.0 mg/kg/day on days –4 to –1 with a haplo-identical familial donor.
Patients from the reference arm received the conventional dosage of i.v. busulfan (3.2 mg/kg) over 3 hours once a day, and all doses of busulfan were calculated using: (1) ABW if this was less than or equal to the ideal body weight (IBW); (2) IBW if ABW was higher than IBW, but within 120% of IBW; or (3) "IBW+0.40×(ABW-IBW)" if ABW exceeded IBW by more than 120% [15]. IBW was calculated using the following equations, where height measured in inches and weight in kilograms: (1) IBW (men)=50+2.3×(height–60); or (2) IBW (women)=45+2.3×(height–60). For patients in the test arm, the dose of i.v. busulfan was calculated using the new dosage equation, based on the results of a population pharmacokinetic model of i.v. busulfan with the aim of achieving a target AUC using a single daily dose of i.v. busulfan (AUCTarget). The AUCTarget of 5924 µM·min was set at a 4-fold the median AUC achieved by 4 times daily i.v. busulfan as reported by a previous study [15]. According to the population pharmacokinetic model for busulfan, the final covariate model of clearance (CL) was as follows: with CL given in liters per hour and ABW in kilograms [18]. Considering the AUCTarget of 5924 µM·min and population pharmacokinetic clearance model for busulfan, patients in the test arm received the appropriate daily dose of 23×ABW0.5 mg over 3 hours once a day to achieve the daily AUCTarget. For both study groups, busulfan was diluted in normal saline to 0.5 mg/mL and infused via a pump through a central venous catheter.
A limited sampling strategy using 2 samples obtained at different times was prospectively implemented during the first cycle of busulfan treatment. Blood samples (5 mL) were taken from all patients at 2 time points with the first busulfan dosing only. Two sampling times were assigned randomly at 3.5, 5, 6, 7 or 22 hours from the start of the 3-hour busulfan infusion. Block randomization, with stratification for the dosage formula and conditioning regimen, was used. Samples were taken from a central venous line using pre-chilled heparin tubes and plasma separation was performed within 60 minutes. Plasma was separated by centrifugation at 2,500 rpm for 10 minutes at 4℃ within 1 hour, placed in cryogenic vials, and stored at –40℃. Plasma busulfan concentrations were measured using a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS), as previously described [18]. Using the percentage deviation of the mean from the true value and the coefficient of variation as measures of accuracy and precision, respectively, intra- and interday accuracies were determined to be 89.96% to 102.28% and 92.79% to 101.14%, respectively. Intra- and interday precision values were determined to be 4.41% to 14.70% and 0.90% to 7.48%, respectively, with five replicates at each concentration level.
The predictive performance of the population model was assessed by calculating the mean prediction error (MPE) and the root mean squared prediction error (RMSE) as follows:
where PRED and OBS were the predicted and observed concentrations, respectively. The MPE describes the bias of the estimates, while the RMSE describes their imprecision.
Using the established final population pharmacokinetic model [18], predicted concentrations of busulfan were calculated for each patient at the available blood sampling times for the given dosage history and demographic data, including sex, age, and body weight. These predictions were obtained by entering the structural model parameters into NONMEM 6.2.0 (GloboMax LLC, Ellicott City, MD, USA) with typical values of pharmacokinetic parameters and no residual errors. The predicted concentrations were compared with the corresponding measured concentrations by calculating bias (MPE) and imprecision (RMSE). A visual predictive check (VPC) was executed using the Perl-speaks-NONMEM (PsN) [23] to evaluate the predictability of the population pharmacokinetic model for busulfan.
The AUC for the daily busulfan dose was predicted for each patient. This predicted AUC (AUCPRED) was calculated as follows: where Dosei was the individual daily amount of busulfan administered and CLBayesian was the maximum a posteriori Bayesian estimate of clearance for the validation patient. NONMEM was used to calculate CLBayesian using the available concentration measurements with the original population parameter estimates as priors. The prediction error of AUC was calculated for each AUCPRED by comparing with the AUCTarget as follows: where AUCTarget was 5924 µM·min. The accuracy and imprecision of AUCPRED from the population model were assessed by calculation of MPE and RMSE. The variability of AUCPRED was compared between the reference arm and the test arm using relative standard error (RSE).
Thirty-seven adult patients were enrolled and received i.v. busulfan on the first day of conditioning therapy for HCT. Patient characteristics are presented in Table 1 and these did not differ significantly between the reference and test arms. A total of 74 busulfan plasma concentrations were measured.
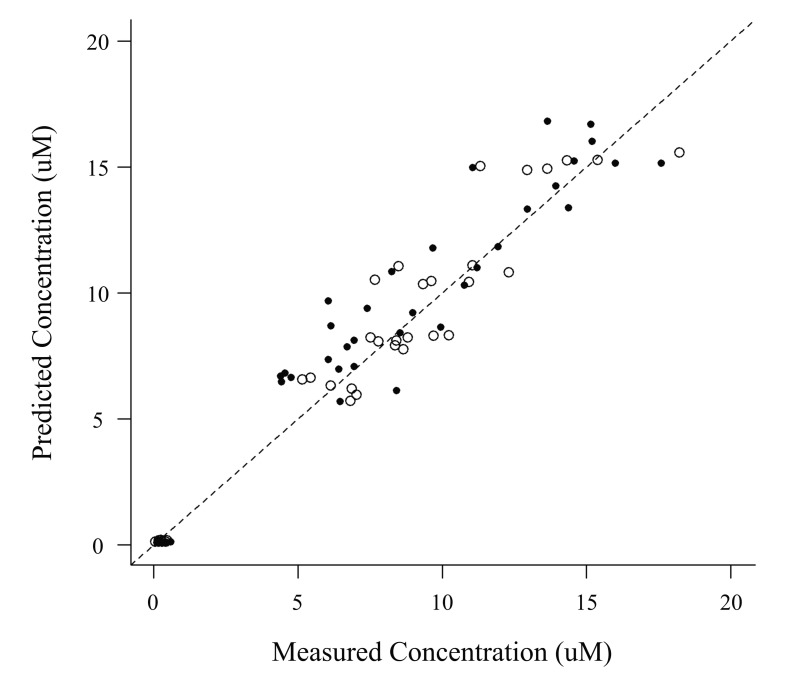
The VPC revealed that the 95% prediction interval of the busulfan concentrations predicted by the population pharmacokinetic model adequately included the observed concentration data (Fig. 1). The relationships between the measured and predicted busulfan concentrations at each sampling time for each patient were well described by a basic diagnostic plot (Fig. 2). The MPE and RMSE values relating to the busulfan concentrations in all of the patients included in this external validation study were 3.73% and 32.37%, respectively. Within the subgroup that received the conventional dosing scheme, the MPE and RMSE values were 9.58% and 33.49%, respectively, while in the subgroup that received the new dosing scheme, these values were -2.44% and 31.15%, respectively.
A box plot of the AUCPRED in the validation group using the population model is shown in Fig. 3. The MPE and RMSE values of AUCPRED in all of the included patients were –2.0% and 17.7%, respectively. Within the conventional dosing scheme subgroup, the RMSE of AUCPRED was 20.6%, this value was 14.0% in the subgroup that received the new dosing scheme (Table 2). The AUCPRED for the new dosage scheme showed less variability than for the conventional dosage scheme (RSE 14.0% vs 21.6%). The MPE of AUCPRED was –5.8% for the conventional dosing scheme and –2.1% using the new dosing scheme.
We have prospectively validated a new dosing scheme for i.v. busulfan given prior to HCT using a limited sampling strategy that included two busulfan concentration-time points for each adult patient. The applicability of this new dosing scheme, based on a population pharmacokinetic model, was externally validated by evaluating its achievement of AUCTarget, while the validity of the population pharmacokinetic model was evaluated for its ability to predict plasma busulfan concentration.
The predictability of busulfan concentrations using a population pharmacokinetic model was evaluated by a simulation-based diagnostic. The VPC revealed the similarity between the measured concentration of busulfan and the simulated concentration. In accordance with these results, the population model was able to provide accurate and precise concentration predictions for all of the patients included in this external validation study.
Regarding the evaluation of busulfan AUC targeting performance, AUCPRED of the test arm with the new dosing scheme showed less variability than that of the conventional dosage regimen (RSE 14.0% vs 21.6%). The optimal AUC range for the oral busulfan 4 times daily regimen in adults and adolescents is 900 to 1500 µM·min [89102425]. A study of 4 times daily i.v. busulfan indicated that 86.2% of patient AUC values were within this range [26]. In our study, an AUC of 5924 µM·min which is a 4 fold median AUC of 4 times daily busulfan, from the learning dataset used to develop the final model was targeted for the new once daily regimen of busulfan. A lower overall survival and progression-free survival at 3 years and an increased nonrelapse mortality at 100 days was reported among patients who received conventional once daily busulfan (3.2 mg/kg), where the busulfan AUC was above 6000 µM·min [17]. Our data showed that patients given conventional dosage had an average AUC values less than 6000 µM·min, while patients given the novel dosing scheme had an average AUC values greater than 6000 µM·min.
A limited sampling strategy based on a Bayesian methodology for i.v. busulfan was developed previously, showing that two samples collected at 2.25 and 6 hours from the start of a 2-hour infusion predicted the AUC with no significant bias and with good precision [27]. In the same study, it was suggested that acceptable estimation of AUC was possible using only one plasma concentration within the first hour post-infusion where sampling after 3 hours post-dosing is less informative. In this study, we could not guarantee the execution of time-fixed blood sampling in the institution, so the limited sampling strategy based on randomly selecting two time points was adopted and our findings indicated that this approach may be sufficient for a reasonable population pharmacokinetic model prediction.
The principal limitation of this study is that the external validation dataset was based on a small and relatively uniform patient population in one of the largest tertiary care hospitals in Korea, so that the value of AUCTarget of this study was not in accord with the result of other study [28]. However we often see the concentrations from the same sample vary more than 20% by the reporting labs. Considering the variability of reported concentrations between assay labs, we decided to use concentrations measured by our in-house laboratory only. Another limitation of included the lack of information of clinical outcomes about toxicity and non-relapse mortality. Therefore, further investigation in a larger population that includes younger patients to evaluate the clinical outcomes is warranted.
In conclusion, this randomized and prospective study demonstrated the validity of a population pharmacokinetic model for busulfan using an external validation method and supported the applicability of the new dosing scheme of busulfan for conditioning therapy for HCT based on a population pharmacokinetic model.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI07C0001).
References
1. Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, Braine HG, Burns WH, Elfenbein GJ, Kaizer H, Mellits D, Sensenbrenner LL, Stuart RK, Yeager AM. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983; 309:1347–1353. PMID: 6355849.

2. Parkman R, Rappeport JM, Hellman S, Lipton J, Smith B, Geha R, Nathan DG. Busulfan and total body irradiation as antihematopoietic stem cell agents in the preparation of patients with congenital bone marrow disorders for allogenic bone marrow transplantation. Blood. 1984; 64:852–857. PMID: 6383499.

3. Blazar BR, Ramsay NK, Kersey JH, Krivit W, Arthur DC, Filipovich AH. Pretransplant conditioning with busulfan (Myleran) and cyclophosphamide for nonmalignant diseases. Assessment of engraftment following histocompatible allogeneic bone marrow transplantation. Transplantation. 1985; 39:597–603. PMID: 3890287.
4. Lucarelli G, Polchi P, Izzi T, Manna M, Delfini C, Galimberti M, Porcellini A, Moretti L, Manna A, Sparaventi G. Marrow transplantation for thalassemia after treatment with busulfan and cyclophosphamide. Ann N Y Acad Sci. 1985; 445:428–431. PMID: 3893278.

5. Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998; 22:439–443. PMID: 9733266.
6. Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y, Galambrun C, Dai Q, Maire P, Jelliffe RW, Aulagner G. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001; 28:743–751. PMID: 11781625.

7. Schuler US, Ehrsam M, Schneider A, Schmidt H, Deeg J, Ehninger G. Pharmacokinetics of intravenous busulfan and evaluation of the bioavailability of the oral formulation in conditioning for haematopoietic stem cell transplantation. Bone Marrow Transplant. 1998; 22:241–244. PMID: 9720736.

8. Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R, Santos GW, Colvin OM. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989; 25:55–61. PMID: 2591002.

9. Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, Lin LS, Devine SM, Geller RB, Heffner LT, Hillyer CD, Holland HK, Winton EF, Saral R. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996; 17:225–230. PMID: 8640171.
10. Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, Soll E, Anasetti C, Bowden R, Bryant E, Chauncey T, Deeg HJ, Doney KC, Flowers M, Gooley T, Hansen JA, Martin PJ, McDonald GB, Nash R, Petersdorf EW, Sanders JE, Schoch G, Stewart P, Storb R, Sullivan KM, Thomas ED, Witherspoon RP, Appelbaum FR. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997; 89:3055–3060. PMID: 9108427.

11. Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, Blume K, Niland J, Palmer JM, Vaughan W, Fernandez H, Champlin R, Forman S, Andersson BS. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002; 8:493–500. PMID: 12374454.

12. Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM, Egeler RM, den Hartigh J, Vossen JM. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005; 35:17–23. PMID: 15502853.

13. Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2002; 8:486–492. PMID: 12374453.

14. Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, Gyonyor E, Andersson BS. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002; 8:468–476. PMID: 12374451.

15. Ryu SG, Lee JH, Choi SJ, Lee YS, Seol M, Hur EH, Lee SH, Bae KS, Noh GJ, Lee MS, Yun SC, Han SB, Lee KH. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007; 13:1095–1105. PMID: 17697972.

16. Bartelink IH, Bredius RG, Ververs TT, Raphael MF, van Kesteren C, Bierings M, Rademaker CM, den Hartigh J, Uiterwaal CS, Zwaveling J, Boelens JJ. Once-daily intravenous busulfan with therapeutic drug monitoring compared to conventional oral busulfan improves survival and engraftment in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008; 14:88–98.

17. Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, Savoie ML, Bahlis NJ, Brown C, Storek J, Andersson BS, Russell JA. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008; 14:220–228. PMID: 18215782.

18. Choe S, Kim G, Lim HS, Cho SH, Ghim JL, Jung JA, Kim UJ, Noh G, Bae KS, Lee D. A simple dosing scheme for intravenous busulfan based on retrospective population pharmacokinetic analysis in korean patients. Korean J Physiol Pharmacol. 2012; 16:273–280. PMID: 22915993.

19. Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997; 37:486–495. PMID: 9208355.

20. Brendel K, Comets E, Laffont C, Laveille C, Mentre F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006; 23:2036–2049. PMID: 16906454.

21. Mentre F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn. 2006; 33:345–367. PMID: 16284919.

22. Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007; 46:221–234. PMID: 17328581.
23. Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004; 75:85–94. PMID: 15212851.

24. Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993; 20:18–25. quiz 26
25. Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G. High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant. 1997; 20:909–913. PMID: 9422468.

26. Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006; 37:345–351. PMID: 16400337.

27. Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006; 57:191–198. PMID: 16133536.

28. McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009; 5:957–969. PMID: 19611402.

Fig. 1
Visual predictive check of the busulfan population pharmacokinetic model in the reference arm and the test arm
The simulated 95% prediction interval is shaded and observations (plasma busulfan concentrations in µM) are depicted as circles and the solid line depicts the model predicted median.
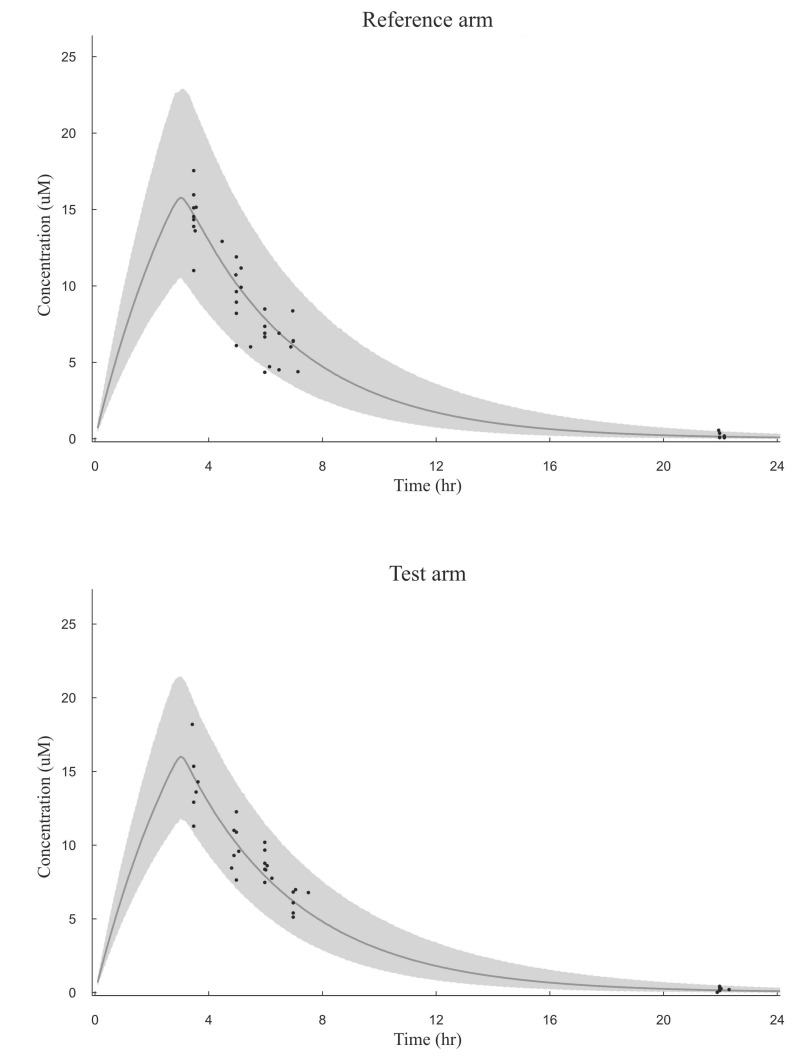
Fig. 2
Predicted versus measured plasma busulfan concentrations in the external validation dataset
Open and closed circles represent values for the patients in the test arm and the reference arm, respectively. The dashed line represents the line of identity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download