Abstract
CD4+CD25+ regulatory T cells (CD4+CD25+ Tregs) have been shown to play a regulatory or suppressive role in the immune response and are possibly relevant to the pathogenesis of autoimmune diseases. In the present study, we attempted to investigate the frequency of CD4+CD25+ Tregs in peripheral blood (PB) of collagen-induced arthritis (CIA) rats during the development of arthritis, to determine whether their frequency is involved in the immunoregulation of this disease. The results showed that normal rats had similar frequencies of CD4+CD25+ Tregs in PB during the experiment time, expressed as a percentage of CD4+CD25+Foxp3+ T cells among the CD4+ T lymphocyte population. In contrast, the frequency of CD4+CD25+Foxp3+ T cells in CIA rats was found to change during the development of arthritis. In CIA rats, there is a significant negative correlation between the frequency of CD4+CD25+Foxp3+ T cells and paw swelling (r=-0.786, p< 0.01). The relationship between the frequency of CD4+CD25+Foxp3+ T and immune activation was not found in normal rats. During the time course, the frequency of CD4+CD25+Foxp3+ T was lower in CIA rats than in normal ones. The data suggest that the frequency of PB CD4+CD25+ Tregs may be a promising marker for arthritis activity.
CD4+CD25+ regulatory T cells (CD4+CD25+ Tregs) characterized by the expression of the IL-2 receptor α-chain (CD25) show a potent immunosuppressive function and contribute to immunologic self-tolerance by suppressing potentially auto-reactive T cells [1]. Deficiency or dysfunction of CD4+CD25+ Tregs may predispose to autoimmune diseases [2]. In recent years, attention has focused on the forkhead box family transcription factor Foxp3, which is critical for both the identification and function of CD4+CD25+ Tregs [3,4].
Some studies support the observation that CD4+CD25+ Tregs play a role in the control of systemic autoimmune disease, and that the loss of this particular cell population enhances the clinical symptoms of chronic arthritis. Morgan et al. used the collagen-induced arthritis (CIA) murine model to demonstrate that the depletion of CD4+CD25+ T cells before CII immunization greatly hastened the onset and severity of arthritis and markedly increased CII-specific antibodies [5]. Adoptively transferring CD4+CD25+T cells could be used therapeutically in CIA despite a lack of reduction in systemic CII-specific T and B cell responses [6]. CD4+CD25+ Tregs and their role in antigen-induced arthritis (AA) have also been investigated [7]. Depletion of CD25+ cells prior to arthritis induction led to an exacerbation of disease with increased cellular and humoral immune responses. Transfer of CD4+CD25+ T cells into immunized mice at the time of induction of AA decreased the severity of disease, but was not able to cure established arthritis. All of these studies showed that the transferred CD4+CD25+ T cells could be traced to the synovial tissue in affected joints, indicating that these cells may modulate inflammation locally.
Although the frequency of CD4+CD25+ Tregs in joint fluid from rheumatoid arthritis (RA) patients was markedly increased in most previous studies [8,9,10,11,12,13], the CD4+CD25+ Tregs counts in the peripheral blood (PB) of RA patients compared to normal controls remain controversial: some studies report normal numbers [9,10]; some report an increase [11]; and others report a decrease [12,13]. Some of this variability may be explained by differences in the labeling and definition of naturally-occurring CD4+CD25+ Tregs. Some studies used CD4+CD25high T cells, whereas others used the CD4+CD25bright T cells. Besides the differences in detection technology, it may be that the PB obtained by these study groups was most likely taken at different clinical stages of RA, providing an explanation for the conflicting results. Thus, although the importance of CD4+CD25+ Tregs in RA has been emphasized, the dynamic ratios of CD4+CD25+ Tregs at different clinical stages have not been fully characterized. In addition, several reports describing the frequency of CD4+CD25+ Tregs in the PB of RA patients did not take Foxp3 into account [8,10,11,12]. In the present study, we chose the model rat collagen-induced arthritis (CIA), and compared the frequency of CD4+CD25+ Tregs in PB of CIA rats during the development of arthritis with healthy controls, in order to determine the correlation between their frequency and disease progression.
Type II collagen (CII) was extracted from chicken sternums and purified by our group, as described before [14]. Fluorescein isothiocyanate (FITC) anti-rat CD4, phycoerythrin (PE) anti-rat CD25, FITC anti-rat IgG2b and PE anti-rat IgG1 antibodies were purchased from Caltag Laboratories and the Regulatory T cell Staining Kit #3 was from eBiosciences.
Male Wistar rats (110~130 g) were obtained from the Experimental Animal Center of Anhui Medical University. They were housed in standard cages at a constant temperature of 22±1℃, relative humidity 55±5% with a natural dark-light cycle for 1 week before the experiment. The animals had free access to food and tap water. The experimental animal protocol was approved by the Anhui Medical University Animal Care and Use Committee.
Collagen-induced arthritis (CIA) was established in Wistar rats as described in our previous paper [15]. Briefly, 2 mg/ml CII was emulsified with an equal volume of IFA. On day 0, rats were injected intradermally with 0.5 ml of the emulsion (containing 0.5 mg of CII) at the base of the tail and other 3~5 sites on the back. Seven days later, a second injection of the emulsion was administered in the same way.
Rats were inspected daily for signs of arthritis characterized by edema and/or erythema in the paws. Bi-hind paw volumes were determined twice a week beginning on the day when arthritic signs were first visible with a volume meter (Shandong Medical Scientific Equipment Station, Shandong, China). The paw swelling (ml) was expressed as an increase in mean bi-hind paw volume by subtracting that at day 0.
At a series of time points post-immunization, peripheral blood was collected from angulus oculi medialisin into heparin-containing tubes. The frequency of CD4+CD25+ Tregs was detected by flow cytometry. Briefly, 100 µl whole blood was lysed with NH4Cl lysing solution, mixed thoroughly, and incubated for 8 min in the dark at 4℃. Samples were centrifuged at 500 g for 10 min. The supernatant was removed and washed twice with cold PBS. Then cells were stained with FITC-conjugated anti-CD4 antibody and PE-conjugated anti-CD25 antibody or appropriate isotype control antibody in the dark for 30 min at 4℃. All antibodies were used at concentrations titrated for optimal staining according to the manufacturer's protocol. Samples were centrifuged at 500 g for 10 min. The supernatant was removed and washed twice with cold PBS. Then, cells were incubated with freshly prepared Fixation/Permeabilization working solution overnight at 4℃. After blocking with Fc block in Permeabilization Buffer, cells were then stained with PE-Cy5-conjugated anti-Foxp3 antibody in the presence of Permeabilization Buffer for 45 min in the dark. The labeled cells were detected immediately on a FACS Calibur (Becton-Dickinson, CA, USA) after washing twice with the Permeabilization Buffer. Analysis of 10,000 lymphocyte events per tube was performed using WinMDI software (Joseph Trotter, Scripps Research Institute, La Jolla, CA).
Data were expressed as (mean±S.E.M) in figures. Significant differences between groups were determined by Student's t-test. For linear regression analysis, Spearman correlation coefficient was calculated. p-values less than 0.05 were considered significant for all statistical tests.
The onset of arthritis appeared on about day 10 after the injection of CII (three days after the second immunization), with a peak onset on about day 17. During the development of arthritis, CIA rats demonstrated significantly more paw swelling than the normal ones (Fig. 1A). Fig. 1B is the photo of a paw of a normal rat on day 17, and Fig. 1C is the photo of a paw of a CIA rat on day 17.
The anti-Foxp3 antibody was used to identify peripheral blood Foxp3-expression lymphocytes in the three-color flow cytometric analysis that also analyzed the CD4 and CD25 expression to correlate the Foxp3 expression, since CD4+ CD25+ Treg cells presumably co-express CD4, CD25 and Foxp3. Gating strategy used to determine the proportion of CD4+CD25+Foxp3+ Tregs was summerized in Fig. 2. Briefly, lymphocytes were gated using an FSC vs. SSC plot (Fig. 2A), followed by a CD4+ gate in a CD4 (FL-1) vs. CD25 (FL-2) plot (Fig. 2B). Then, in CD4+ T cells, Tregs were discriminated according to their CD25 (FL-2) and Foxp3 (FL-3) expression, by which CD4+CD25+Foxp3+ cells are considered as Tregs (Fig. 2C). In our study, Foxp3-expressing cells were barely detected in the CD4-negative lymphocytes, indicating that Foxp3 is predominantly expressed in Fig. 1. the CD4+ T cells in the PB. Therefore, CD4+CD25+Foxp3+ T cells could well represent CD4+CD25+ Tregs.
To investigate changes in the number of CD4+CD25+ Tregs as the arthritis progressed, dynamic analysis of the profiles of Foxp3-expressing cells was carried out at a series of time points. The proportion of CD4+CD25+Foxp3+ T cells in normal rats was almost the same during the study period, while it was changing in CIA rats during the progression of arthritis. During the first 2 weeks post-immunization, the percentage of CD4+CD25+Foxp3+ T cells in PB of CIA rats was only a little lower than normal rats, with the difference between the two groups not being significant. However, when the inflammation develops, the ratio was significantly decreased in the immunized group compared to normal rats. On day 17, when the arthritis was most serious, as calculated by paw swelling, the frequency of CD4+CD25+Foxp3+ T cells in CIA rats was the lowest. After that, the ratio rebounded, but is still lower than the normal level (Fig. 3). Therefore, CIA rats showed a significant decrease in CD4+CD25+ Treg percentage during arthritis development when compared to healthy rats.
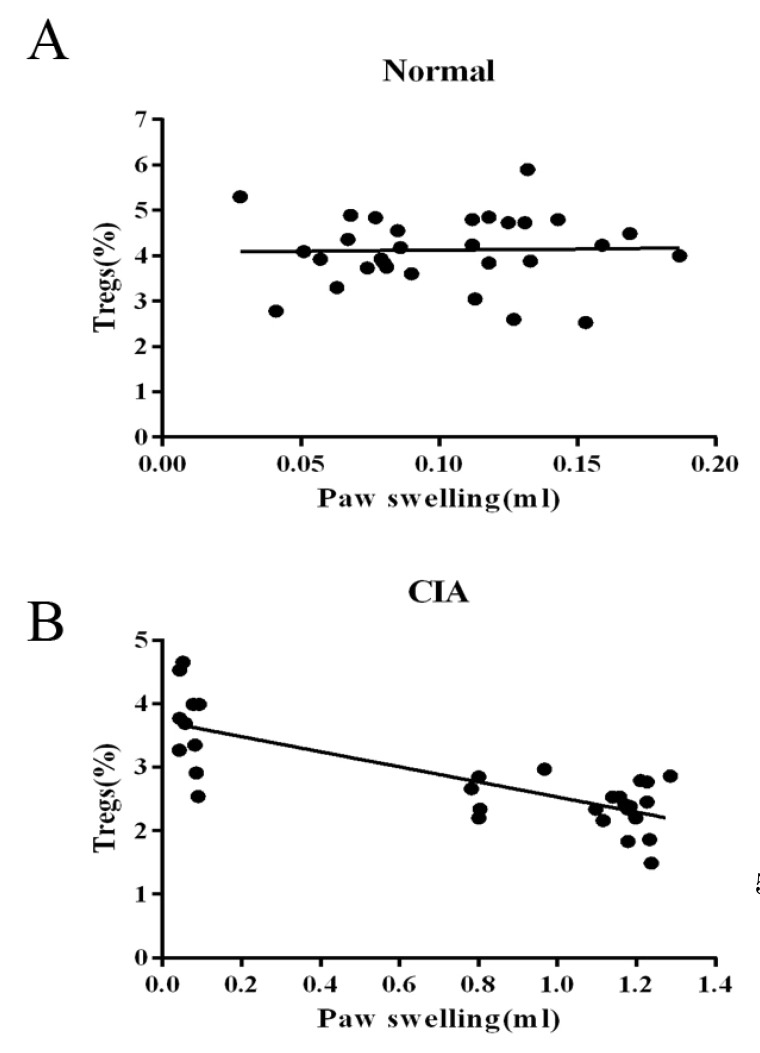
We then determined by simple linear regression whether the frequency of PB CD4+CD25+ Tregs is involved in the immunoregulation of this disease. As the sample size of flow cytometric analysis was smaller than that used for the paw volume measurement, we only chose data from the rats that had been determined by both of the two targets. As shown in Fig. 4, the relationship between the frequency of CD4+CD25+Foxp3+ T cells populations in CD4+ T cells and immune activation was not found in normal rats, while the frequency was negatively correlated with paw swelling with statistical significance (r=-0.786) in CIA rats.
CIA is an autoimmune-mediated polyarthritis that shares important similarities with RA in histology and immunology [16,17]. The immune reaction of CIA rats is regulated by complex mechanisms, which are incompletely understood, but regulatory T cells are able to suppress the activation of CD4+ T cells [18]. CD4+CD25+ Tregs are a subset of T cells which are involved in peripheral immune tolerance by suppressing auto-reactive T cells. Their role in autoimmune disease, which occurs through a breakdown of tolerance, is of particular interest in trying to ascertain the mechanisms of disease progression [19]. Several reports have investigated the expression of CD4+CD25+ Tregs, but the relationship between the frequency of CD4+CD25+ Tregs and disease progression has not been fully described, especially among CIA rats. In the present study, we studied the correlation of the alternation of CD4+CD25+ Tregs in the PB of CIA rats and disease progression.
The identification of CD4+CD25+ Tregs during ongoing immune responses is complicated, as activation markers such as CD25 are also expressed on activated effector T cells [20,21]. Over the past few years, several studies have demonstrated that the intracellular marker Foxp3 is expressed in CD4+CD25+ Tregs and therefore it appears to be of great significance for both the identification and function of CD4+CD25+ Tregs [22]. Additionally, Foxp3 expression is limited to CD4+CD25+ Tregs in mice and could not be induced in CD4+CD25- effector cells upon activation [3]. Thus, we characterized the CD4+CD25+ Tregs as the percentage of CD4+CD25+Foxp3+ T cells among CD4+ T cells. Our results showed that the frequency of CD4+CD25+Foxp3+ T cells was the same as that in the controls 2 week post-CII-immunization. At the chronic stage of inflammation, the percentage in CIA rats significantly decreased, with the expression of Foxp3 showing a gradual decrease along with arthritis progression. As shown in Fig. 3, the frequency of CD4+ CD25+Foxp3+ T cells in CIA rats was significantly lower than that of normal rats after day 17. We also observed that the down-regulation of the frequency of CD4+ CD25+ Tregs was significantly correlated with paw swelling in CIA rats (r=-0.786, p<0.01), suggesting that the continuous arthritis may be due to the decrease of CD4+CD25+ Tregs over the course of disease progression. In contrast, no relationship between the frequency of CD4+CD25+ Tregs and immune activation was seen in normal rats (p>0.05). We also found that the mRNA and protein level of Foxp3 was decreased in the spleens of CIA rats than in normal controls and could be improved by leflunomide both in vivo and in vitro [23]. These results are consistent with our hypothesis of the involvement of CD4+CD25+Tregs in CIA progression and with the findings of several published studies, which showed an increase of CD4+CD25+Tregs in the therapeutic effect of anti-arthritic drugs on CIA [24,25,26,27].
Taken together, our data indicate that CD4+CD25+ Tregs might be involved in the immune modulation during the course of CIA, which enhances the current knowledge of mechanisms of the immune disorder in CIA. It should be noted, however, that much of the data regarding CD4+CD25+ Tregs has been generated using animal models. Thus, future studies will examine the dynamic frequency of CD4+CD25+ Tregs at different clinical stages to provide new insights into the autoimmune genesis and therapeutic strategies of RA. It is hoped that by understanding the role of CD4+CD25+ Tregs in autoimmunity, a reliable therapy may be developed to cure the disease.
ACKNOWLEDGEMENTS
The authors are indebted to Dr. Jun Zhang from the University of Science and Technology of China (USTC) for his technical assistance in flow cytometry. This research was funded by the National Natural Science Foundation of China (NSFC 81301531), Shanghai College Young Investigator Oversea Research Program, the Innovation Program of the Shanghai Municipal Education Commission (NO.12YZ049), and the Young Teacher Training Program in the Universities in Shanghai (shjdy036). The Opening Project of Shanghai Key Laboratory of Orthopaedic Implant (KFKT2011003) and the Excellent Young Development Program of Shanghai Ninth People's Hospital supported part of this work.
References
1. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155:1151–1164. PMID: 7636184.
2. Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014; 20:69–74. PMID: 24317118.

3. Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007; 7:305–310. PMID: 17380159.

4. Mu J, Tai X, Iyer SS, Weissman JD, Singer A, Singer DS. Regulation of MHC class I expression by Foxp3 and its effect on regulatory T cell function. J Immunol. 2014; 192:2892–2903. PMID: 24523508.

5. Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003; 48:1452–1460. PMID: 12746920.

6. Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005; 52:2212–2221. PMID: 15986351.

7. Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Bräuer R. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005; 7:R291–R301. PMID: 15743476.
8. Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003; 33:215–223. PMID: 12594850.

9. Möttönen M, Heikkinen J, Mustonen L, Isomäki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005; 140:360–367. PMID: 15807863.
10. Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005; 62:312–317. PMID: 16179019.

11. van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004; 50:2775–2785. PMID: 15457445.

12. Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmström V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004; 6:R335–R346. PMID: 15225369.
13. Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford). 2006; 45:1210–1217. PMID: 16571607.

14. Kong W, Li J, Wang TY, Huang LY. Establishment and evaluation of collagen-induced arthritis model in rats. Acta Univ Med Anhui. 2008; 43:3–7.
15. Wang TY, Li J, Jin Z, Wu F, Zhou Q. Inhibitory effect of TGF-β1 on NO production in peritoneal macrophages from collagen-induced arthritis rats involving the LPS-TLR4 pathway. Mol Med Rep. 2013; 8:1143–1148. PMID: 23970162.

16. Vierboom MP, Jonker M, Bontrop RE, 't Hart B. Modeling human arthritic diseases in nonhuman primates. Arthritis Res Ther. 2005; 7:145–154. PMID: 15987497.
17. Kim YO, Hong SJ, Yim SV. The efficacy of shikonin on cartilage protection in a mouse model of rheumatoid arthritis. Korean J Physiol Pharmacol. 2010; 14:199–204. PMID: 20827333.

18. Langier S, Sade K, Kivity S. Regulatory T cells: the suppressor arm of the immune system. Autoimmun Rev. 2010; 10:112–115. PMID: 20807589.

19. Anderson AE, Isaacs JD. Tregs and rheumatoid arthritis. Acta Reumatol Port. 2008; 33:17–33. PMID: 18344919.
20. Nolte-'t Hoen EN, Boot EP, Wagenaar-Hilbers JP, van Bilsen JH, Arkesteijn GJ, Storm G, Everse LA, van Eden W, Wauben MH. Identification and monitoring of effector and regulatory T cells during experimental arthritis based on differential expression of CD25 and CD134. J Leukoc Biol. 2008; 83:112–121. PMID: 17928458.
21. Kim JM, Joo HG. Immunostimulatory Effects of β-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes. Korean J Physiol Pharmacol. 2012; 16:225–230. PMID: 22915986.
22. Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014; 15:580–587. PMID: 24728351.

23. Wang TY, Li J, Li CY, Jin Y, Lü XW, Wang XH, Zhou Q. Leflunomide induces immunosuppression in collagen-induced arthritis rats by upregulating CD4+CD25+ regulatory T cells. Can J Physiol Pharmacol. 2010; 88:45–53. PMID: 20130738.
24. Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Vasoactive intestinal peptide induces CD4+,CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum. 2006; 54:864–876. PMID: 16508968.

25. Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells. Ann N Y Acad Sci. 2007; 1110:630–640. PMID: 17911478.

26. Gonzalez-Rey E, Chorny A, O'Valle F, Delgado M. Adrenomedullin protects from experimental arthritis by down-regulating inflammation and Th1 response and inducing regulatory T cells. Am J Pathol. 2007; 170:263–271. PMID: 17200199.

27. Su D, Shen M, Gu B, Wang X, Wang D, Li X, Sun L. 99 Tc-methylene diphosphonate improves rheumatoid arthritis disease activity by increasing the frequency of peripheral γδ T cells and CD4+ CD25+ Foxp3+ Tregs. Int J Rheum Dis. 2014; doi: 10.1111/1756-185X.12292. [Epub ahead of print].
Fig. 1
Paw swelling of CIA rats. The paw swelling was expressed as an increase in mean bi-hind paw volume by subtracting that at day 0. (A) Paw swelling during arthritis progress in CIA rats. (B) Photo of the hind paw of a normal rat on day 17. (C) Photo of the hind paw of a CIA rat on day 17. Values are expressed as the means±SEM for ten animals in each group. Compared with normal group, ##p<0.01.
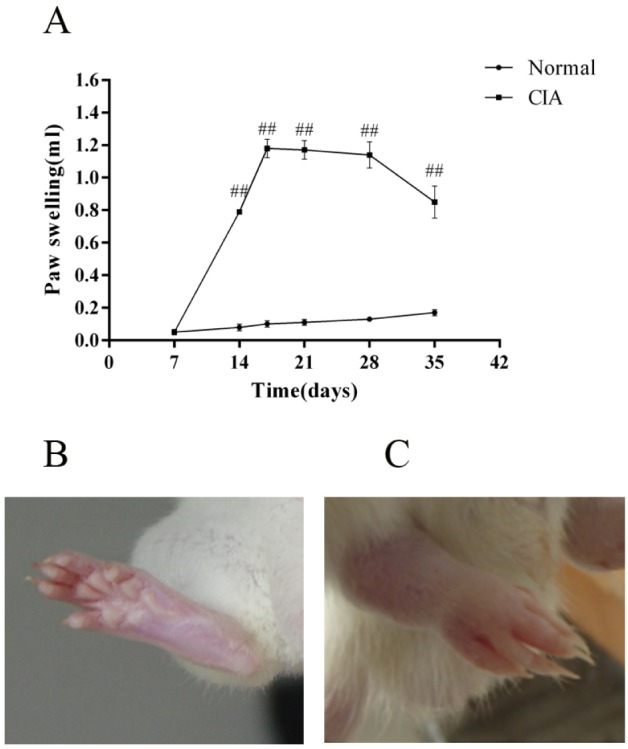
Fig. 2
Gating strategy used to determine the proportion of CD4+CD25+Foxp3+ Tregs. Erythrocytes were lysed with NH4Cl lysing solution. Cells were stained with FITC-conjugated anti-CD4 antibody and PE-conjugated anti-CD25 antibody. After permeabilization, cells were stained with PE-Cy5-conjugated anti-Foxp3 antibody. The labeled cells were detected on a FACS Calibur. Lymphocytes were gated using an FSC vs. SSC plot (A), followed by a CD4+ gate in a CD4 (FL-1) vs. CD25 (FL-2) plot (B). Then, in CD4+ T cells, Tregs were discriminated according to their CD25 (FL-2) and Foxp3 (FL-3) expression, by which CD4+CD25+Foxp3+ cells are considered as Tregs (C).
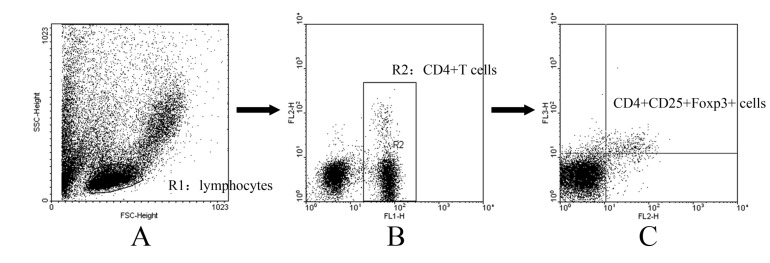
Fig. 3
Proportion of peripheral blood CD4+CD25+ Tregs during the arthritis progress. (A) Flow cytometry of peripheral blood CD4+CD25+ Tregs of normal and CIA rats at different time points. (B) Proportion of peripheral blood CD4+CD25+ Tregs during arthritis progress. Values are expressed as the means±SEM for five animals in each group. Compared with normal group, #p<0.05, ##p<0.01.
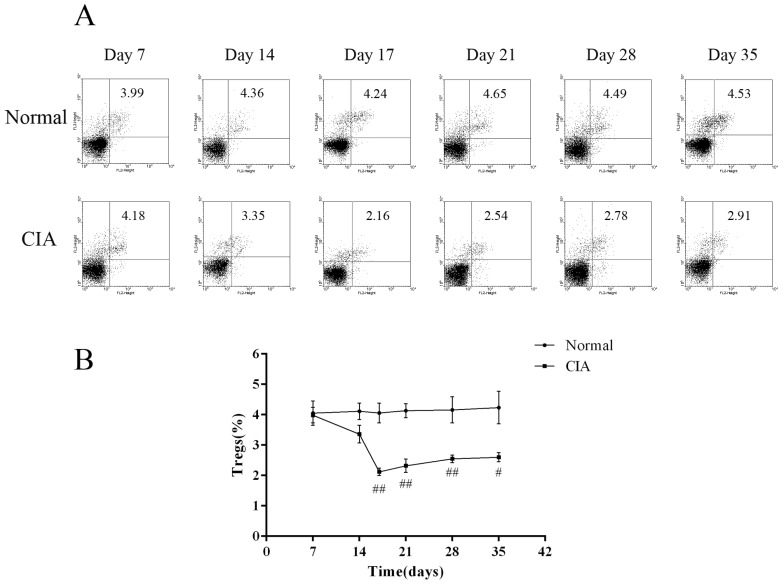
Fig. 4
Correlation between the frequency of CD4+CD25+Foxp3+ Tregs and paw swelling. Linear regression was used to analyze the correlation between the frequency of peripheral blood CD4+CD25+ Foxp3+ T cells and paw swelling during arthritis progress. (A) The frequency of CD4+CD25+ Tregs was not correlated with paw swelling in normal rats. (B) The frequency of CD4+CD25+ Tregs was negatively correlated with paw swelling with statistical significance in CIA rats (r=-0.786).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download