Abstract
K+ outward currents in the outer hair cells (OHCs) of circling mice (homozygous (cir/cir) mice), an animal model for human deafness (DFNB6 type), were investigated using a whole cell patch clamp technique. Littermate heterozygous (+/cir) mice of the same age (postnatal day (P) 0 -P6) were used as controls. Similar slow rising K+ currents were observed in both genotypes, but their biophysical and pharmacological properties were quite different. The values of Vhalf for activation were significantly different in the heterozygous (+/cir) and homozygous (cir/cir) mice (-8.1±2.2 mV, heterozygous (+/cir) mice (n=7) and -17.2±4.2 mV, homozygous (cir/cir) mice (n=5)). The inactivation curve was expressed by a single first order Boltzmann equation in the homozygous (cir/cir) mice, while it was expressed by a sum of two first order Boltzmann equations in the heterozygous (+/cir) mice. The K+ current of homozygous (cir/cir) mice was more sensitive to TEA in the 1 to 10 mM range, while the 4-AP sensitivities were not different between the two genotypes. Removal of external Ca2+ did not affect the K+ currents in either genotype, indicating that the higher sensitivity of K+ current to TEA in the homozygous (cir/cir) mice was not due to an early expression of Ca2+ activated K+ channels. Our results suggest that the K+ outward current of developing homozygous (cir/cir) mice OHCs is different in both biophysical and pharmacological aspects than that of heterozygous (+/cir) mice.
Previous findings have shown that the expression of K+ channels is developmentally regulated in murine cochlear hair cells. From embryonic day 14.5 to P 30, delayed rectifier K+ current (IK,emb, IK,neo) [1], inward rectifier K+ current (IK1) [2], K+ current activated at negative potentials (IK,n) [3], delayed rectifier K+ current (IK,s), and Ca2+ activated K+ current (IK,f) [4] are sequentially expressed in murine cochlear hair cells. The timely expression of K+ channels is important for the functional maturation of cochlear hair cells [1,3,4], which raises a possibility that early stage K+ channel expression might be different in the cochlear hair cells of genetically abnormal animals, such as mouse models for human deafness.
The circling mouse is a mouse model for human deafness (DFNB6 type) [5,6]. The most notable pathologic findings of the circling mice are their completely degenerated organ of Corti, which can occur as early as P21, as well as markedly reduced cellularity of the spiral ganglion neurons [6]. A scanning electron microscope study demonstrated that the stereocilliary defects of OHCs were observable as early as P10 and were more severe in the OHCs than in the inner hair cells (IHCs) at P18 in circling mice [6].
It has been reported that progressive hearing loss is paralleled by a selective degeneration of OHCs in KCNQ4 K+ channel knockout mice (Kcnq4-/- mice) [7], which raises a possibility that the alteration of K+ channel expression might affect the degeneration of OHCs in circling mice. However, the types of K+ channels expressed prior to degeneration in the OHCs of circling mice have not been reported. The aim of this study was to investigate the expression and characteristics of the K+ channels in circling mice OHCs during the first postnatal week.
Female heterozygous (+/cir) mice were mated with male homozygous (cir/cir) mice (circling mice), and their offspring were used for this study. The circling mice strain has been maintained for more than 10 generations by breeding between female heterozygous (+/cir) mice and their male siblings (homozygous (cir/cir) mice) at the Animal Facility of Dankook University since 2007. The data presented were obtained from pups between P0 and P6. Genotypes were assessed by polymerase chain reaction analysis according to our previous report [8]. The Dankook University Institutional Animal Care and Use Committee (DUIAC) approved this study.
After the mice received deep anesthesia with isoflurane, their cochleae were removed and dissected in an ice-cold solution composed of (in mM): NaCl (124), KCl (5), KH2PO4 (1.25), glucose (10), NaHCO3 (26), CaCl2 (2), MgSO4 (1.3), and sucrose (20). The pH was 7.4 when aerated with 95% O2 and 5% CO2 and the osmolarity was about 305 mOsm. After removing the bony part and modiolus, the dissected cochleae were transferred to a submersion-type chamber mounted on an upright microscope and immobilized under a nylon mesh fixed to a stainless steel ring. The chamber was perfused continuously with a same solution used during preparation.
Whole cell currents were recorded from OHCs located in the middle turn of the cochlea. All experiments were performed at room temperature using an EPC-8 (HEKA, Lambrecht, Germany) amplifier. Electrodes (3~5 MΩ) were filled with a solution containing (in mM): K-gluconate (108), EGTA (0.6), KCl (5), HEPES (10), Na2GTP (0.3), MgATP (1), KOH (30), sucrose (47), and QX 314 (5). All chemicals except QX 314 (Tocris) were purchased from Sigma Chemicals Co., unless otherwise stated. Except fast capacitance cancellation at the cell-attached stage, series resistance was not compensated and no corrections were made for the small liquid junction potentials (<5 mV). The data were filtered at 5 kHz (EPC-8, HEKA), digitized at 10 kHz, and stored in the computer via a home-made program (R-Clamp 1.23) for offline analysis. The stored data were analyzed using Clampfit 9.0 (Molecular Devices), Origin 7.0 (Origin Lab) and SPSS V. 21 (IBM). Data were expressed as the mean±SEM. An independent t-test and repetitive-measured ANOVA were used for comparisons. Null hypotheses of no difference were rejected if p-values were <.05.
Typical examples of K+ currents recorded from neonatal OHCs of heterozygous (+/cir) and homozygous (cir/cir) mice are shown in Figs. 1A and 1B, respectively. Depolarizing voltage steps from -100 mV to 40 mV (holding potential: -60 mV) evoked slowly inactivating outward K+ currents in the OHCs of both genotypes, which were similar to that reported previously (a delayed rectifier-type K+ current, IK,neo [1,4], for neonatal cells). Almost no inward currents were elicited by the hyperpolarizing voltage steps from -60 mV to -100 mV in both genotypes (Figs. 1A and 1B). Using the current amplitudes measured at the end of voltage steps (200 ms) and cell capacitance measured from current transients in response to 2 mV step pulses, current densities were calculated for analysis. In heterozygous (+/cir) and homozygous (cir/cir) mice, cell capacitance was 5.5±0.1 pF (n=66) and 5.7±0.1 pF (n=118). The two values were not significantly different. The developmental changes were not significantly different either in two groups (Fig. 1D). The current densities measured at 40 mV were 227.1±18.9 pA/pF (n=16) in heterozygous (+/cir) mice and 238.9±14.3 pA/pF (n=22) in homozygous (cir/cir) mice (Fig. 1C). The two values were not different significantly. Reversal potentials, determined by applying a conditioning pulse to 0 mV, followed by a series of test pulses from -120 mV to -50 mV with a 10 mV increment (data not shown), were -60.5±1.3 mV in the heterozygous (+/cir) mice (n=7) and -57.8±0.9 mV in the homozygous (cir/cir) mice (n=5). The two values were not significantly different.
Figs. 2A and 2B show the voltage-dependent activation and inactivation of K+ currents. Inactivation curves were obtained by measuring the peak currents recorded at a potential of 30 mV after conditioning pulses (1s) from the holding potential of -60 mV. The conditioning pulses were from -100 mV to more depolarized voltage levels in 10 mV increments. Normalized peak currents were plotted against the different conditioning potentials and fitted by a modified first-order Boltzmann equation: y=A2+(A1-A2)/{1+exp(V-Vhalf/S)} where y is the normalized peak current, A2 is the minimal normalized peak current, A1 is the maximal normalized peak current, Vhalf is the potential of half-maximal inactivation, V is the commanding potential, and S is the voltage sensitivity of inactivation.
Normalized peak currents obtained from homozygous (cir/cir) mice were fitted with a single first-order Boltzmann equation (Fig. 2B). Vhalf was -59.6±4.0 mV and the slope factor was 10.2±0.4 mV (n=6). In heterozygous (+/cir) mice, the inactivation curve was expressed by the sum of two inactivation curves (69% contribution of curve 1 (-100 mV~-20 mV) and 31% contribution of curve 2 (-20 mV~20 mV)), fitted by a first-order Boltzmann equation (Fig. 2A). The first Vhalf between -100 mV and -20 mV was -59.5±2.3 mV and the slope factor was 9.4±1.1 mV (n=6) and the second Vhalf, between -20 mV and 20 mV, was 1.2±1.7 mV and the slope factor was 4.9±0.9 mV (n=6).
The activation of K+ outward currents was analyzed by calculating conductance (peak current/(Vcommand-Vholding)) at each commanding potential (Vholding=-60 mV). Voltage steps were applied from -60 mV with 10 mV increment to 40 mV. The activation curves in Figs. 2A and 2B were obtained by plotting the normalized conductance values against the commanding potentials. Data were fitted by the same Boltzmann equation used in the inactivation analysis. The values of Vhalf for heterozygous (+/cir) and homozygous (cir/cir) mice were -8.1±2.2 mV (n=7) and -17.2±4.2 mV (n=5), respectively. The values of the slope factors for heterozygous (+/cir) and homozygous (cir/cir) mice were 23.1±2.9 mV (n=7) and 25.6±2.5 mV (n=5), respectively. The values of Vhalf were significantly different (independent t-test, p<0.05).
The above results indicated that some of the biophysical properties of the K+ current of homozygous (cir/cir) mice were different than those of the heterozygous (+/cir) mice. Therefore, we tested whether the sensitivity to K+ channel blockers was also different. Tetraethylammonium (TEA) and 4-aminopyridine (4-AP) were selected for the experiment. K+ currents were activated by a series of voltage steps from -50 mV to 40 mV (holding potential: -60 mV) and the current amplitudes were measured at the end of the voltage steps (200 ms) for analysis. For TEA, a range of 1 to 100 mM were tested and for 4-AP, a range of 0.1 to 10 mM was tested. An equal concentration of NaCl was replaced with TEA-chloride when a high concentration of TEA was used.
In heterozygous (+/cir) mice, the K+ current recorded at 40 mV was reduced to 78.4±2.8% (n=5), 56.6±2.7% (n=5), and 13.9±2.1% (n=5) of the control by 1 mM, 10 mM, and 100 mM TEA, respectively (Fig. 3A). In homozygous (cir/cir) mice, the corresponding reductions were 62.7±4.8% (n=5), 38.7±3.8% (n=5), and 20.1±2.3% (n=5), respectively (Fig. 3B). The extent of inhibition by 1 mM and 10 mM TEA was significantly greater in homozygous (cir/cir) mice (repetitive-measured ANOVA and independent t-test, p<0.05).
4-AP also reduced the amplitude of the K+ current in a dose-dependent manner. In heterozygous (+/cir) mice, the K+ current recorded at 40 mV was reduced to 52.8±6.0% (n=6), 23.0±5.5% (n=6), and 11.4±2.1% (n=6) of the control by 0.1 mM, 1 mM, and 10 mM 4-AP, respectively (Fig. 4A). In homozygous (cir/cir) mice, the corresponding reductions were 46.3±6.0% (n=5), 26.0±4.0% (n=5), and 10.0±2.4% (n=5), respectively (Fig. 4B). At all concentrations, the extent of inhibition was not significantly different between the two groups.
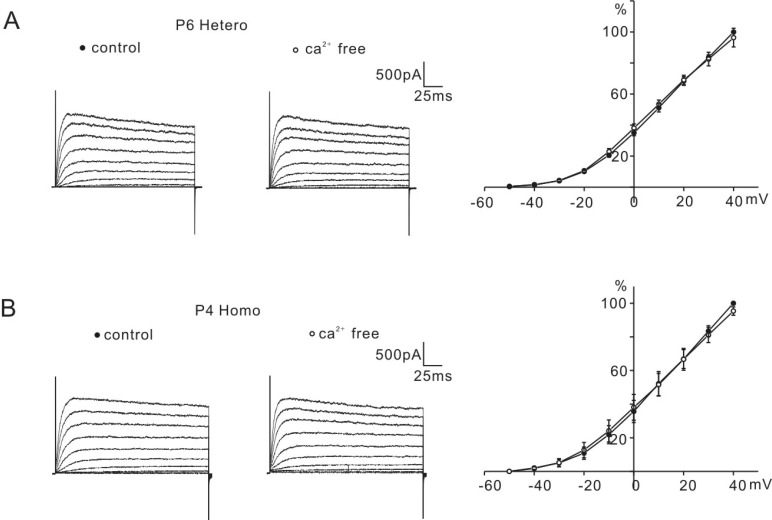
Though a Ca2+-activated K+ current has not been reported in P0-P6 mice OHCs, because a significant difference in the percentage reduction between the two groups was observed only with the application of TEA, we tested whether it was due to an early expression of the Ca2+-activated K+ channels in homozygous (cir/cir) mice. For that purpose, the amplitude of the K+ current was measured before and after the removal of external Ca2+. In both groups, the removal of the external Ca2+ did not affect the K+ current amplitude (Fig. 5), which suggested that the greater inhibition of K+ currents in homozygous (cir/cir) mice than in heterozygous (+/cir) mice might not be due to the early expression of Ca2+-activated K+ channels in homozygous (cir/cir) mice.
Currently, only two types of K+ currents, a transiently expressed inward rectifier (IK1) [2] and a delayed rectifier-type outward (IK,neo) [1,3], have been reported in P0-P6 mice OHCs. During this period, the K+ outward currents observed in heterozygous (+/cir) and homozygous (cir/cir) mice are quite similar to a delayed rectifier K+ current (IK,neo) reported in CD-1 mice, with respect to its slow activation and partial inactivation during voltage steps.
The biophysical properties of the outward K+ currents of the P0-P6 homozygous (cir/cir) mice and the heterozygous (+/cir) mice were not same, but were similar to the IK,neo reported in CD-1 mice IHCs or OHCs. The Vhalf for activation in the homozygous (cir/cir) mice and heterozygous (+/cir) mice were about -17 mV and -8 mV, but it was -32 mV (OHC) [3] and -35 mV (IHC) in CD-1 mice [1]. This difference might come from the differences in recording conditions and the method of analysis (such as series resistance compensation, membrane and junction potential correction, and analysis of instantaneous tail currents) in addition to the cell difference. However, the difference of Vhalf for activation between two genotypes was apparent.
The inactivation curves were also different. In homozygous (cir/cir) mice OHCs, the inactivation curve was expressed with a single first-order Boltzmann equation, while it was expressed with a sum of two first-order Boltzmann equations in the heterozygous (+/cir) mice OHCs. The inactivation curve of heterozygous (+/cir) mice OHCs was similar to that reported in CD-1 mice IHCs [1]. As the inactivation curve for the OHCs of normal P0-P6 mice has not been reported, we do not know whether the inactivation curve expressed with a sum of two first-order Boltzmann equations is typical in P0-P6 mice OHCs. However, considering that the OHCs of P0-P6 mice are in the process of development and new K+ channels will be expressed soon, the two component inactivation curve observed in the heterozygous (+/cir) mice OHCs and CD-1 mice IHCs might be more typical. Whether this single component inactivation curve reflects a developmental delay or immaturity will require more developmental study.
4-AP sensitivities of both genotypes were quite different than those reported in embryonic mice OHCs [9] or P0-P6 mice IHCs [1]. It has been reported that 4-AP application reduced K+ currents with the peak currents being more affected than the steady-state levels [9], and that there is no difference in the percentage of current reduction between the application of 1 and 10 mM 4-AP [1], which was not the case in the heterozygous (+/cir) or homozygous (cir/cir) mice in the present study. In both genotypes, 4-AP reduced K+ outward currents dose-dependently in the range of 0.1 to 10 mM and 4-AP reduced both the peak and steady-state currents to a similar extent, although the peak current was inhibited a little more strongly (Fig. 4). Moreover, the extent of the inhibition by 10 mM 4-AP was about 30% of the IK,neo of P0-P6 CD-1 mice IHCs, but it was almost 90% in homozygous (cir/cir) or heterozygous (+/cir) mice. This potent inhibition by 4-AP is similar to that observed in adult OHCs [10,11]. Although 4-AP is of limited use in attempts to determine the identity of the underlying currents, it might be suggested that the K+ outward currents observed in heterozygous (+/cir) and homozygous (cir/cir) mice seem to be different than those reported in other embryonic OHCs or in CD-1 mice IHCs.
In contrast to the effect of 4-AP, the TEA sensitivities of both genotypes were quite close to those reported in P0-P6 mice IHCs [1]. With respect to the reported half-blocking concentration (5.6 mM) in P0-P6 CD-1 mice IHCs, the TEA sensitivity of homozygous (cir/cir) mice in the present study seems to be close to a normal response. We do not know why TEA sensitivities are different from those of 4-AP in the two genotypes.
Though TEA sensitivities of both genotypes were similar at the concentration of 100 mM, they were statistically different in the range of 1 to 10 mM and this different sensitivity might not be due to the different composition of the K+ outward currents. Among the known K+ channels which are expected to be expressed during early development, possible candidates for outward K+ current might be a small conductance Ca2+-activated K+ channel (SK) and a large conductance Ca2+-activated K+ channel. However, the expression time and TEA sensitivity of these channels are not in line with our data. First, small conductance Ca2+-activated K+ channel (SK) is observed only from the beginning of the second postnatal week [12] and SK is known to be resistant to external TEA [13]. Second, the large conductance Ca2+-activated K+ current, IK,f, in developing IHCs is sensitively blocked by submillimolar TEA (IC50=0.3±0.03 mM), expressed after the onset of hearing (around 10~12 days after birth) and shows much faster activation [4]. The only Ca2+-activated K+ current showing similar characteristics with respect to activation kinetics and sensitivity to TEA is an additional Ca2+-activated K+ current reported in the IHCs of CD-1 mice aged between P14 and P27 [14], but this current has never been reported in the OHCs of P0-P6 mice. Moreover, we have not observed any changes in K+ current amplitude by external Ca2+ removal. Thus, the different TEA sensitivities might be considered to be characteristics of the K+ currents in the two genotypes.
The purpose of this study was to investigate the expression and characteristics of K+ outward currents in the OHCs of homozygous (cir/cir) mice. We do not know how this K+ current contributes to the gradual degeneration of the OHCs starting from the second postnatal week. Further investigation of the developmental changes in K+ channel expression might be needed to elucidate their role in OHC degeneration.
ACKNOWLEDGEMENTS
The present research was conducted by the research fund of Dankook University in 2013.
References
1. Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003; 548:383–400. PMID: 12588897.

2. Marcotti W, Géléoc GS, Lennan GW, Kros CJ. Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea. Pflugers Arch. 1999; 439:113–122. PMID: 10651007.

3. Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999; 520 Pt 3:653–660. PMID: 10545133.
4. Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998; 394:281–284. PMID: 9685158.

5. Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, Lee JR, Kim HT, Suh JG, Kim TY, Ryoo ZY. Circling mouse: possible animal model for deafness. Comp Med. 2001; 51:550–554. PMID: 11924819.
6. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, Cho KI, Hong SH. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 127:244–251. PMID: 17364360.

7. Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006; 25:642–652. PMID: 16437162.
8. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 28:13003–13007. PMID: 19036993.

9. Helyer RJ, Kennedy HJ, Davies D, Holley MC, Kros CJ. Development of outward potassium currents in inner and outer hair cells from the embryonic mouse cochlea. Audiol Neurootol. 2005; 10:22–34. PMID: 15486441.

10. Mammano F, Ashmore JF. Differential expression of outer hair cell potassium currents in the isolated cochlea of the guinea-pig. J Physiol. 1996; 496:639–646. PMID: 8930832.

11. Liang GH, Järlebark L, Ulfendahl M, Moore EJ. Mercury Hg2+ suppression of potassium currents of outer hair cells. Neurotoxicol Teratol. 2003; 25:349–359. PMID: 12757831.
12. Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol. 2004; 560:691–708. PMID: 15331671.

13. Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996; 19:150–154. PMID: 8658599.
14. Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca2+ on Ca2+-activated K+ currents in mature mouse inner hair cells. J Physiol. 2004; 557:613–633. PMID: 15064328.
Fig. 1
Whole cell K+ outward currents of P0-P6 mice OHCs. Current traces recorded from P5 heterozygous (+/cir) and P3 homozygous (cir/cir) mouse are shown in A and B. Currents were elicited by depolarizing voltage steps from -100 mV to 40 mV with 10 mV increment (holding potential: -60 mV). Voltage protocol is shown above the current traces. Current density-voltage curves are shown in C (filled circle: heterozygous (+/cir) mouse, hollow circle: homozygous (cir/cir) mouse). Cell capacitance changes from P0 to P6 are shown in D (filled circle: heterozygous (+/cir) mouse, hollow circle: homozygous (cir/cir) mouse). Cell capacitances are 5.09±0.4 (P2, n=13), 5.7±0.3 (P3, n=15), 5.7±0.3 (P4, n=15), 5.3±0.2 (P5, n=16), and 5.3±0.3 (P6, n=12) in heterozygous (+/cir) mice. They are 5.9±0.3 (P1, n=12), 5.9±0.2 (P3, n=21), 5.5±0.2 (P4, n=26), 5.6±0.1 (P5, n=30), and 5.9±0.1 (P6, n=29) in homozygous (cir/cir) mice.
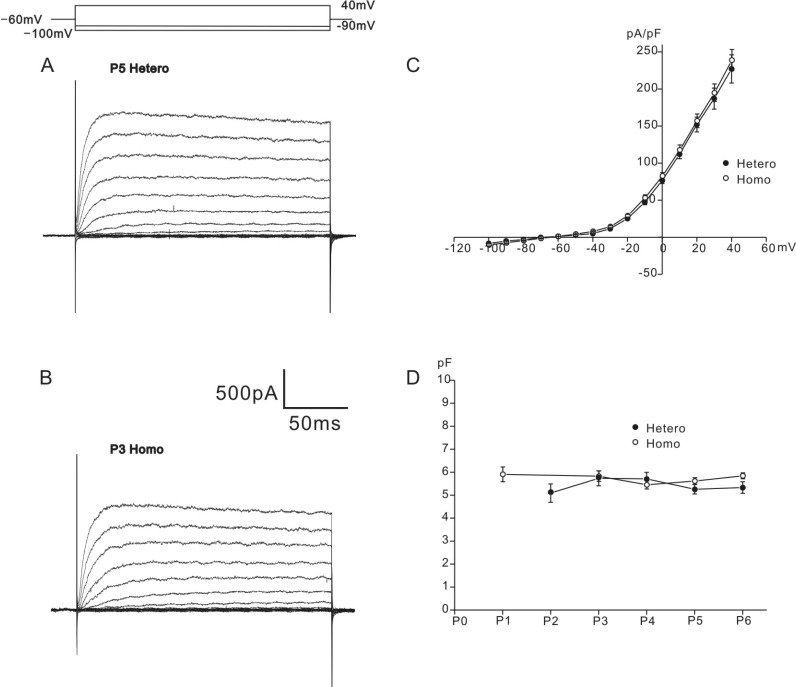
Fig. 2
Inactivation (square) and activation (circle) curves for the K+ currents. Activation and inactivation curves for heterozygous (+/cir) mice and homozygous (cir/cir) mice are shown in A and B, respectively. Current trace examples for the inactivation curve fit are shown to the left of the plots. The activation curves in Figs. 2A and 2B were obtained by plotting the normalized conductance values against the commanding potentials.
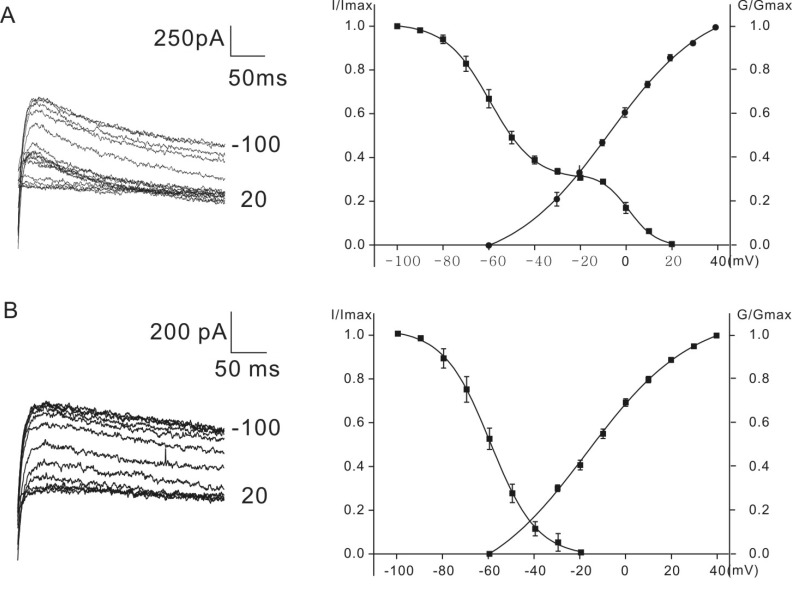
Fig. 3
Effect of TEA on K+ currents. The effects of TEA (1 to 100 mM) on K+ currents are shown in A (P5 heterozygous (+/cir) mouse) and B (P3 homozygous (cir/cir) mouse). Currents were elicited by depolarizing voltage steps from -50 mV to 40 mV (the holding potential was -60 mV). Reduced currents were normalized (I/Imax) with the peak currents at 40 mV before drug application. Normalized currents - voltage curves are shown by the current traces (C). Marks above the current traces indicate the measuring points and TEA concentrations.
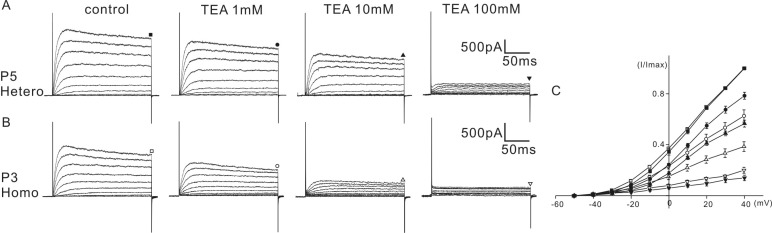
Fig. 4
Effect of 4-AP on K+ currents. The effects of 4-AP (0.1 to 10 mM) on K+ currents are shown in A (P4 heterozygous (+/cir) mouse) and B (P5 homozygous (cir/cir) mouse). Currents were elicited by depolarizing voltage steps from -50 mV to 40 mV (the holding potential was -60 mV). Reduced currents were normalized (I/Imax) with the peak currents at 40 mV before drug application. Normalized currents - voltage curves are shown by the current traces (C). Marks above the current traces indicate the measuring points and 4-AP concentrations.
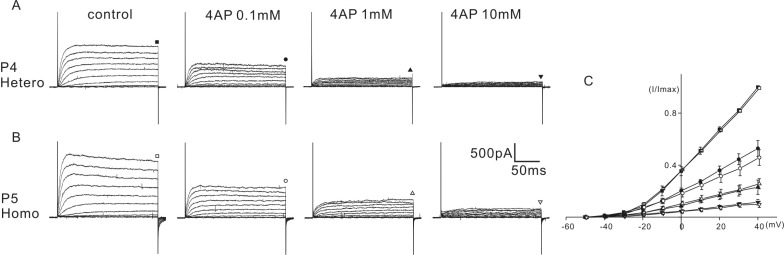
Fig. 5
Effect of external Ca2+ removal on K+ currents. Nominal Ca2+-free solution did not changes the K+ currents of P6 heterozygous (+/cir) mouse (A) or P4 homozygous (cir/cir) mouse (B). Normalized current-voltage curves are shown by the current traces (filled circle represents control and hollow circle represents Ca2+-free).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download