Abstract
Attenuated functional exercise capacity in elderly and diseased populations is a common problem, and stems primarily from physical inactivity. Decreased function and exercise capacity can be restored by maintaining muscular strength and mass, which are key factors in an independent and healthy life. Resistance exercise has been used to prevent muscle loss and improve muscular strength and mass. However, the intensities necessary for traditional resistance training to increase muscular strength and mass may be contraindicated for some at risk populations, such as diseased populations and the elderly. Therefore, an alternative exercise modality is required. Recently, blood flow restriction (BFR) with low intensity resistance exercise (LIRE) has been used for such special populations to improve their function and exercise capacity. Although BFR+LIRE has been intensively studied for a decade, a comprehensive review detailing the effects of BFR+LIRE on both skeletal muscle and vascular function is not available. Therefore, the purpose of this review is to discuss previous studies documenting the effects of BFR+LIRE on hormonal and transcriptional factors in muscle hypertrophy and vascular function, including changes in hemodynamics, and endothelial function.
The loss of muscle strength and mass with age, also known as sarcopenia, is a common detrimental issue leading to a decline in functional capacity, mobility, and endurance in the elderly [1,2,3]. In addition to sarcopenia, cardiovascular disease (CVD) is also a common disease that results from decreased cardiac and vascular smooth muscle elasticity in the elderly [4]. Also, advancing age may cause a reduction in central arterial compliance, which is highly related to endothelial dysfunction [5,6,7]. However, age-related arterial stiffening and endothelial dysfunction may be restored by lifestyle modifications such as regular exercise training [8,9,10].
Previously, several studies have reported a reduction in arterial stiffness following an 8-week course of aerobic exercise training [10,11]. This is worth noting as aging is related to a decline in aerobic fitness [12]. Although aerobic fitness is an important component of healthy aging, aerobic exercise is not sufficient to lessen sarcopenia in the aging muscle. Hence resistance exercise training should be included as a means to maintaining muscle mass. According to the American College of Sports Medicine (ACSM), optimizing muscular strength and hypertrophy can be achieved through moderate to high intensities of resistance exercise that utilize 8~10 upper and lower body exercises [13]. These exercises should target major muscle groups 2~3 d·wk-1 at a training intensity of more than 65% of the subject's one-repetition maximum [13]. However, the elderly and individuals who have chronic diseases may find it difficult to perform regular exercise at moderate to high intensities. Although the beneficial effects of resistance exercise on skeletal muscle function have been well documented, the increase in blood pressure during resistance exercise in older adults who have high blood pressure and other forms of CVD are still problematic. In addition, some studies have reported that chronic resistance exercise training (RET) may increase central and peripheral arterial stiffness and sympathetic activity [14,15,16,17]. Therefore, it is imperative to propose an alternative training protocol aimed at increasing muscular strength and mass, but without the detrimental effects on cardiovascular function in older adults.
Recently, blood flow restriction (BFR) combined with low intensity resistance exercise (LIRE) has been suggested as a useful exercise protocol to gain muscular strength and mass without an increase in blood pressure. BFR+LIRE is typically performed by placing a narrow compressive cuff around an appendicular limb and inflating it immediately prior to exercise. The pressure of the cuff is around super systolic (150~350 mmHg), and the exercise intensity is 25~45% of maximum voluntary contraction (MVC). BFR+LIRE may be used for a variety of exercise modalities including both isometric and isotonic contractions including chest press, arm curl, leg extension, squat, etc. Improvements in muscular strength and mass in older people have been reported in many studies utilizing BFR+LIRE [18,19,20,21,22,23,24,25,26]. Moreover, several studies reported that BFR+LIRE training acutely reduced arterial stiffness along with improving muscular strength [8,27]. The primary benefit of using BFR+LIRE is that it improves muscular strength and cardiovascular function concurrently. Additionally, it is suitable for special populations such as the elderly and diseased populations who cannot perform traditional resistance or aerobic exercise training [28].
This review will mainly highlight the impact of BFR+LIRE, first on skeletal muscle hypertrophy, including hormonal and transcriptional factors, and second on vascular function including endothelial function and cardiovascular autonomic modulation.
Muscle hypertrophy and increased strength following single high-intensity resistance exercise bouts are generally thought to be associated with the recruitment of high threshold motor units [29,30]. Recruitment of these motor units results in an increase in mechanical stress [31,32], endocrine responses [33], and metabolite accumulation [34]. For example, large, acute increases in growth hormone (GH) immediately after exercise has been theorized to stimulate the secretion of insulin like growth factors (IGFs) leading to increased protein synthesis and ultimately muscle hypertrophy [35]. To this point, BFR+LIRE has been popularized for nearly a decade because of its ability to augment endocrine response when compared to LIRE without BFR [18,36]. Previous studies have reported that in comparison to high intensity resistance exercise, BFR+LIRE evokes similar endocrine response and muscular adaptations such as an increase in muscular hypertrophy and mass, albeit in a shorter exercise duration [18,37,38]. In addition to these chronic positive effects, Pierce and colleagues reported an acute 9-fold increase in serum GH from baseline to the cessation of knee extension exercise at 20% maximum voluntary contraction (MVC) with BFR [39]. Others have reported GH increases of up to 290 times baseline values [18,37,38,40,41], with the GH responses following BFR+LIRE being similar to, or even higher than those reported during high intensity resistance exercise of intensities at about 70% 1RM [35,42]. It has been suggested that a key role in the GH release during exercise is played by lactate accumulation, a theory supported by the fact that individuals who lack the enzyme myophosphorylase (those who do not show an increase in blood lactate during exercise) display a blunted GH response [43].
The production of IGF-1 is also increased with GH. In fact, IGF-1 increases protein synthesis and activates satellite cells, all of which causes myofibril hypertrophy [44]. In addition, muscular hypertrophy has occurred with the viral overexpression of IGF-1 [45]. Therefore, BFR+LIRE seemed to be not only a useful stimulator to increase muscular GH, but also a safe exercise modality for the elderly. Unfortunately, the effects of BFR+LIRE on hormonal growth factors in diseased populations such as those with spinal cord injury, CVD, and metabolic syndrome have not been well documented. Therefore, further studies to confirm whether these diseased populations demonstrate a similar hormonal response to BFR+LIRE are warranted.
Protein synthesis is a pivotal factor governing skeletal muscle hypertrophy, and is triggered by transcriptional factors including up-regulation of mRNA in mammalian target of rapamycin (mTOR) pathways [46]. Increased signaling for skeletal muscle hypertrophy, as identified by up regulation of mTOR and IGF-1 measurements are evident within 24 h after a single bout of resistance exercise in human skeletal muscle [47,48,49]. Indeed, this rapid time course allows for the acute evaluation of the hypertrophic potential of BFR+LIRE.
Recently, Fujita and Yasuda reported that muscle protein synthesis and ribosomal S6 kinase 1 (S6K1) phosphorylation are increased after a single bout of BFR+LIRE [50]. These acute changes in transcriptional factors associated with muscle hypertrophy after BFR+LIRE may be explained by the up-regulation of hypertrophy-associated genes including phosphoinositide 3- kinase (PI3K), protein kinase B (AKT), and mTOR. Drummond et al. demonstrated that the short duration of hypoxia and reperfusion induced by BFR promotes cell survival and cell growth adaptations within skeletal muscle by the activation of the mTOR pathway [46]. In addition, up-regulation of vascular endothelial growth factor (VEGF), considered an important modulator in vasculogenesis and angiogenesis, is essential for hypertrophy and can be stimulated by hypoxia and lactate accumulation [46,51,52,53]. Moreover, an increase in the rate of angiogenesis has been described when lactate levels are elevated, thus eliciting a greater hypertrophic stimulus [54]. According to such findings, lactate accumulation derived from BFR-induced hypoxia is a potent stimulus for muscle hypertrophy. Taken together, the above referred studies points towards the conclusion that BFR+LIRE appears to be an effective method for inducing an increase in hypertrophy related transcriptional factors in skeletal muscle via induction of hypoxia compared to resistance exercise without BFR.
The impact of BFR with RT on hemodynamic parameters is well documented. Previously, studies suggested that moderate to heavy resistance exercise involving large muscle groups, especially those utilizing isometric contractions, evoke a significant increase in mean arterial pressure as both systolic and diastolic pressure are elevated [55,56]. Furthermore, when compared with younger counterparts, older individuals exhibit a similar or even greater pressor response to isometric exercise [57,58]. As the intensity of the resistance exercise is increased, there are concurrent increases in blood pressure, which significantly increase pulse pressure amplification estimated by the elevation of aortic pulse wave velocity (a surrogate of aortic stiffness) in humans [14,59,60]. These findings highlight the influence of exercise intensity on the biophysical and elastic properties of the heart's large central arteries and the possible relationship of exercise intensity on the cardiovascular risk of a patient with increased CVD risk factors [61].
While the exercise-induced pressor response is considered inherent to resistance exercise, it is important to note that BFR can elicit significantly greater changes in blood pressure as compared to traditional exercise [62]. Renzi, et al. cautioned the prescription of BFR for those with compromised cardiovascular systems such as the elderly and those with CVD. Their findings demonstrated an exaggerated heart rate response (cardiac reactivity) to BFR over non-BFR, despite a similar cardiac output in young, healthy adults [62]. This led to significantly greater cardiac work and myocardial oxygen demand after BFR+ walk training [63]. However, contradicting results have been shown in elite athletes, as BFR positively impacted stroke volume [64].
Due to the robust changes in blood flow dynamics during BFR, it is important to understand the peripheral and central blood flow changes that take place and how these changes may impact the safety of BFR. In this regard, Takano et al. demonstrated a similar response in BFR as compared to a work-matched non-BFR control in total peripheral resistance and ankle brachial index [65]. Blood flow changes post-exercise have demonstrated varying results following isometric exercise, with both increases [66] and decreases [67] being reported. Post exercise blood flow has also been found to be increased to a larger extent following chronic BFR as compared to traditional resistance exercise [68]. The authors suggested that the increase in post exercise blood flow may be due to increased venous compliance, which enables increased arterial inflow post exercise [68]. Additional explanations have pointed to oxygen restriction and vasoactive metabolite accumulation (e.g. H+, CO2, lactate, etc.) as precursors to post exercise blood flow [69].
In sum, the peripheral blood flow response following BFR+exercise appears to be similar to that of regular exercise and should not be a cause for concern regarding the safety of this training modality.
Although the impact of BFR+LIRE on vascular function has not been well documented, BFR+LIRE does not appear to negatively affect blood vessel function as determined by the arterial compliance of large and small arteries [27,70]. Walk training with BFR has also been shown to induce positive effects on both muscle hypertrophy and carotid arterial compliance, concurrently [23]. In contrast, a study suggested that flow-mediated vasodilation, an indicator of endothelial function, decreased following BFR+walk training. This decreased endothelial function was explained by ischemia-reperfusion injury [62]. Also, Credeur et al. suggested that 12 weeks of BFR+handgrip exercise training impaired endothelial function in brachial arteries [71]. The authors explained the functional impairment by the stimulation of endothelin I, induced by BFR, which increased retrograde flow in working muscle [71]. Although endothelial function in the vasculature is important for understanding the mechanisms of alterations in function following BFR+LIRE, there are limited studies and inconsistent results investigating this phenomenon. Therefore, further studies are warranted to understand how BFR+LIRE affects vascular function.
The impact of LIRE on autonomic function in diseased populations such as hypertensive and heart failure patients, has indicated an improvement of parasympathetic nerve activation [72,73]. However, LIRE does not improve parasympathetic nerve activity to the same degree in healthy young and old individuals. Rather, a higher intensity of resistance exercise training must be implemented to achieve similar benefits as that of the diseased population performing LIRE [74,75].
Blood flow restriction can result in an enhancement of response of the autonomic nervous system (ANS) as determined by means of experimental occlusion. In this procedure, the effects of leg blood flow restriction on the ANS were investigated using power spectral analysis of heart rate variability [76]. Recently, several studies reported that BFR might increase sympathetic nervous system activity [70] by stimulating the ANS, possibly through input from barareceptors to regulate blood pressure against external perturbations [77]. Baroreflex sensitivity and autonomic function are important factors of vasomotor control, and researchers suggested that the high pressures on the limbs induced by BFR cuff stimulates baroreflex, leading to vasoconstriction and an increased blood pressure response [16,78]. Iida and colleagues reported that application of BFR alone on the legs reduced blood flow significantly, including cardiac output, due to the pooling of venous blood and the reduction of femoral arterial blood inflow [70]. This BFR induces the retention of blood flow in the lower extremities, and causes subsequent hemodynamic changes including autonomic nervous activities. The subsequent adaptation for lower body negative pressure has been shown to be a useful method to prevent orthostatic intolerance after bed rest. Therefore, BFR may be a useful method to stimulate the adequate activity of autonomic function, which may ultimately help elderly patients avoid the risk of fainting and falling.
There is lack of standardized exercise prescription for BFR exercise, including pressure, exercise duration, and intensity. In general, BFR studies inflate pressure cuffs' pressures to a value higher than brachial diastolic blood pressure (cuff pressure of 100~200 mmHg) in order to restrict venous blood flow [21,67,79]. Cuff pressure is also inflated higher than brachial systolic blood pressure (cuff pressure of ≥300 mmHg) to stimulate a restriction in arterial blood flow [38,80,81] with 20~45% of MVC. However, there are no standardized cuff pressures or exercise intensities in the BFR+LIRE studies, which results in varied outcomes. Cook et al. suggested that continuous venous BFR (BFR during whole exercise session) with 20% of MVC is the most demanding and potent stimulator for muscle growth [82]. Initially, Fales and colleagues investigated the different effects between venous BFR using low cuff pressure, and complete arterial BFR using high cuff pressure in dog gastrocnemius/plantaris muscles [83]. This study suggested that both complete arterial and venous BFR results in reduction of oxygen utilization in the local muscle [83],with a similar degree of potential skeletal muscle hypertrophy. In addition, Iida et al. suggested that venous BFR caused by low occlusion pressure (100 mmHg) results in a similar decrease in resting femoral artery blood flow, and similar hypoxic condition in leg muscles as compared to 250 mmHg [70]. Also, Takarada et al. suggested that LIRE with a venous BFR pressure of 100 mmHg is sufficient compression to stimulate plasma lactate accumulation and hypoxia in working muscle [37]. However, Karabulut et al. suggested that higher initial restrictive pressures (cuff pressure of ≥300 mmHg) significantly decreased muscle tissue oxygenation and lead to an increased deoxyhemoglobin which is a stimulator of muscle hypertrophy [84]. Furthermore, Figueroa et al. suggested that the LIRE with venous BFR pressure of 100 mmHg was not enough to induce the extra stimulation compared to LIRE alone [79]. The inconsistent BFR cuff pressure in previous studies is a confounding factor, which leads to irregular results in BFR+LIRE studies. This certainly warrants further study to identify the optimal cuff pressure for BFR.
The selection of cuff size is an additional factor of importance for BFR. In fact, Rossow et al. demonstrated that wider (13.5 cm) BFR cuffs are required to induce greater tissue hypoxia, which is a stimulator of muscular hypertrophy. Wider cuffs also resulted in elevation of heart rate, brachial and central blood pressures, perceived effort, and pain as compared to a narrower (5.0 cm) BFR cuff during knee extension exercise [85]. However, studies from Japanese scientists suggest that a narrow cuff size is required to induce capillary refill, which causes mild tissue hypoxia. Narrow cuff size does not induce severe hypoxic induced skeletal muscle tissue damage [18,21,46]. Therefore, the inconsistent methods utilized in previous studies warrant further investigation to identify the optimal cuff pressure and size that induces proper hypoxic conditions in working muscle.
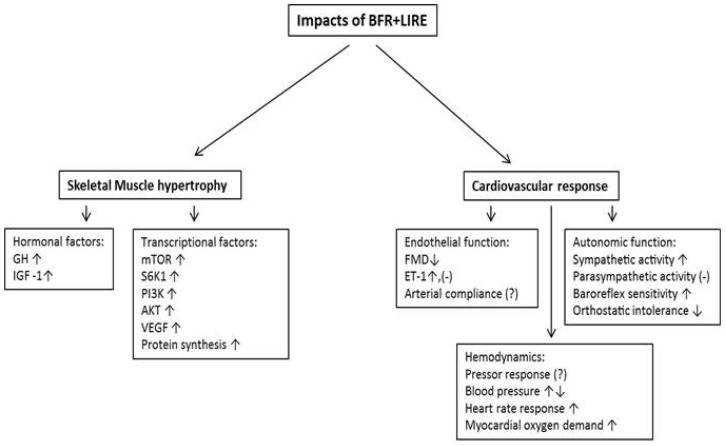
In conclusion, the combination of BFR+LIRE has been shown to enhance muscle hypertrophy and strength without the need of employing high exercise intensity. In addition, the physiological responses including endocrine, transcription, blood pressure, and hemodynamics to this style of training are similar to, or even greater than conventional resistance or aerobic exercise alone (Fig. 1). However, the effects of BFR+LIRE on vascular function have not been well elucidated, which warrants further investigation. Furthermore, the safety of BFR+LIRE training has not been established in diseased populations. Even with these unsolved issues, many studies confirm that BFR+LIRE training improves muscular strength and mass more efficiently (I.e. lower exercise intensity) compared to exercise without BFR. Thus, BFR+LIRE training is not only a useful method for improving or maintaining the age associated decreases in functional capacity but also an efficient exercise modality for rehabilitation by promoting positive changes in muscle while only requiring low intensity exercise in humans.
References
2. Fabre JM, Wood RH, Cherry KE, Su LJ, Cress ME, King CM, deVeer MJ, Ellis R, Jazwinski SM. Louisiana Healthy Aging Study. Age-related deterioration in flexibility is associated with health-related quality of life in nonagenarians. J Geriatr Phys Ther. 2007; 30:16–22. PMID: 19839176.

3. Savinainen M, Nygård CH, Korhonen O, Ilmarinen J. Changes in physical capacity among middle-aged municipal employees over 16 years. Exp Aging Res. 2004; 30:1–22. PMID: 14660330.

4. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachialankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009; 27:2022–2027. PMID: 19550355.

5. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994; 24:471–476. PMID: 8034885.

6. Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001; 37:529–534. PMID: 11230330.
7. Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab. 2001; 11(Suppl):S97–S104. PMID: 11915936.

8. Otsuki T, Takanami Y, Aoi W, Kawai Y, Ichikawa H, Yoshikawa T. Arterial stiffness acutely decreases after whole-body vibration in humans. Acta Physiol (Oxf). 2008; 194:189–194. PMID: 18462269.

9. Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000; 529(Pt 1):263–271. PMID: 11080267.

10. Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998; 18:127–132. PMID: 9445266.

11. Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009; 32:1531–1535. PMID: 19509011.

12. Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009; 169:1781–1787. PMID: 19858436.

13. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009; 41:459–471. PMID: 19127177.
14. Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, Tanaka H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens. 2005; 18:930–934. PMID: 16053989.
15. Kawano H, Tanimoto M, Yamamoto K, Sanada K, Gando Y, Tabata I, Higuchi M, Miyachi M. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp Physiol. 2008; 93:296–302. PMID: 17911355.

16. Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004; 110:2858–2863. PMID: 15492301.
17. Heffernan KS, Sosnoff JJ, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Fractal scaling properties of heart rate dynamics following resistance exercise training. J Appl Physiol (1985). 2008; 105:109–113. PMID: 18450986.

18. Abe T, Sakamaki M, Fujita S, Ozaki H, Sugaya M, Sato Y, Nakajima T. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. J Geriatr Phys Ther. 2010; 33:34–40. PMID: 20503732.
19. Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985). 2010; 108:1199–1209. PMID: 20150565.

20. Yasuda T, Fukumura K, Uchida Y, Koshi H, Iida H, Masamune K, Yamasoba T, Sato Y, Nakajima T. Effects of low-load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. J Gerontol A Biol Sci Med Sci. 2014; [Epub ahead of print].

21. Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol (1985). 2006; 100:1460–1466. PMID: 16339340.

22. Karabulut M, Abe T, Sato Y, Bemben MG. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol. 2010; 108:147–155. PMID: 19760431.

23. Ozaki H, Miyachi M, Nakajima T, Abe T. Effects of 10 weeks walk training with leg blood flow reduction on carotid arterial compliance and muscle size in the elderly adults. Angiology. 2011; 62:81–86. PMID: 20682613.

24. Ozaki H, Miyachi M, Nakajima T, Abe T. Muscle volume and strength and arterial compliance after walk training with blood flow reduction in elderly women. J Am Geriatr Soc. 2010; 58:1597–1598. PMID: 20942877.
25. Ryushi T, Kumagai K, Hayase H, Abe T, Shibuya K, Ono A. Effect of resistive knee extension training on postural control measures in middle aged and elderly persons. J Physiol Anthropol Appl Human Sci. 2000; 19:143–149.

26. Thiebaud RS, Loenneke JP, Fahs CA, Rossow LM, Kim D, Abe T, Anderson MA, Young KC, Bemben DA, Bemben MG. The effects of elastic band resistance training combined with blood flow restriction on strength, total bone-free lean body mass and muscle thickness in postmenopausal women. Clin Physiol Funct Imaging. 2013; 33:344–352. PMID: 23701116.

27. Fahs CA, Rossow LM, Thiebaud RS, Loenneke JP, Kim D, Abe T, Beck TW, Feeback DL, Bemben DA, Bemben MG. Vascular adaptations to low-load resistance training with and without blood flow restriction. Eur J Appl Physiol. 2014; 114:715–724. PMID: 24375201.

28. Rees SS, Murphy AJ, Watsford ML. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: a randomized clinical trial. Phys Ther. 2008; 88:462–470. PMID: 18218826.

29. Mackintosh SF, Goldie P, Hill K. Falls incidence and factors associated with falling in older, community-dwelling, chronic stroke survivors (>1 year after stroke) and matched controls. Aging Clin Exp Res. 2005; 17:74–81. PMID: 15977453.
30. Mackintosh SF, Hill K, Dodd KJ, Goldie P, Culham E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil. 2005; 19:441–451. PMID: 15929514.

31. Häkkinen K. Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int J Sports Med. 1993; 14:53–59. PMID: 8463025.

32. Häkkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol (1985). 1993; 74:882–887. PMID: 8458810.
33. Kraemer RR, Hollander DB, Reeves GV, Francois M, Ramadan ZG, Meeker B, Tryniecki JL, Hebert EP, Castracane VD. Similar hormonal responses to concentric and eccentric muscle actions using relative loading. Eur J Appl Physiol. 2006; 96:551–557. PMID: 16369814.

34. Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol. 1987; 391:1–11. PMID: 3443943.

35. Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005; 35:339–361. PMID: 15831061.

36. Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev. 2009; 37:78–85. PMID: 19305199.

37. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol (1985). 2000; 88:2097–2106. PMID: 10846023.
38. Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002; 86:308–314. PMID: 11990743.

39. Pierce JD, Goodyear-Bruch C, Hall S, Clancy RL. Effect of dopamine on rat diaphragm apoptosis and muscle performance. Exp Physiol. 2006; 91:731–740. PMID: 16644796.

40. Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000; 32:2035–2039. PMID: 11128848.

41. Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol (1985). 2000; 88:61–65. PMID: 10642363.
42. Ploutz LL, Tesch PA, Biro RL, Dudley GA. Effect of resistance training on muscle use during exercise. J Appl Physiol (1985). 1994; 76:1675–1681. PMID: 8045847.

43. Godfrey RJ, Whyte GP, Buckley J, Quinlivan R. The role of lactate in the exercise-induced human growth hormone response: evidence from McArdle disease. Br J Sports Med. 2009; 43:521–525. PMID: 18184755.

44. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985). 2001; 91:534–551. PMID: 11457764.

45. Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol (1985). 2006; 100:1778–1784. PMID: 16439513.

46. Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008; 40:691–698. PMID: 18317375.

47. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL Jr, Urban RJ. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001; 280:E383–E390. PMID: 11171591.

48. Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol (1985). 2005; 98:482–488. PMID: 15465884.

49. Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005; 146:1772–1779. PMID: 15618350.
50. Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci. 2009; 27:479–489. PMID: 19253083.

51. Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999; 126:1149–1159. PMID: 10021335.

52. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999; 5:623–628. PMID: 10371499.

53. Semsarian C, Sutrave P, Richmond DR, Graham RM. Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem J. 1999; 339:443–451. PMID: 10191278.

54. Hunt TK, Aslam R, Hussain Z, Beckert S. Lactate, with oxygen, incites angiogenesis. Adv Exp Med Biol. 2008; 614:73–80. PMID: 18290316.

55. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985). 1985; 58:785–790. PMID: 3980383.

56. Rowell L. Cardiovascular control. New York: Oxford University press;1993.
57. Bogaard HJ, Woltjer HH, Dekker BM, van Keimpema AR, Postmus PE, de Vries PM. Haemodynamic response to exercise in healthy young and elderly subjects. Eur J Appl Physiol Occup Physiol. 1997; 75:435–442. PMID: 9189732.

58. Smolander J, Aminoff T, Korhonen I, Tervo M, Shen N, Korhonen O, Louhevaara V. Heart rate and blood pressure responses to isometric exercise in young and older men. Eur J Appl Physiol Occup Physiol. 1998; 77:439–444. PMID: 9562295.

59. DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J Appl Physiol (1985). 2005; 98:2287–2291. PMID: 15718412.

60. Tanaka M, Sugawara M, Ogasawara Y, Izumi T, Niki K, Kajiya F. Intermittent, moderate-intensity aerobic exercise for only eight weeks reduces arterial stiffness: evaluation by measurement of stiffness parameter and pressure-strain elastic modulus by use of ultrasonic echo tracking. J Med Ultrason (2001). 2013; 40:119–124. PMID: 23565047.

61. Sharman JE, McEniery CM, Campbell RI, Coombes JS, Wilkinson IB, Cockcroft JR. The effect of exercise on large artery haemodynamics in healthy young men. Eur J Clin Invest. 2005; 35:738–744. PMID: 16313249.

62. Renzi CP, Tanaka H, Sugawara J. Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sports Exerc. 2010; 42:726–732. PMID: 19952840.

63. Ives DT, Calcus A, Kalluri S, Strelcyk O, Sheft S, Lorenzi C. Effects of noise reduction on AM and FM perception. J Assoc Res Otolaryngol. 2013; 14:149–157. PMID: 23180229.

64. Park S, Kim JK, Choi HM, Kim HG, Beekley MD, Nho H. Increase in maximal oxygen uptake following 2-week walk training with blood flow occlusion in athletes. Eur J Appl Physiol. 2010; 109:591–600. PMID: 20544348.

65. Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005; 95:65–73. PMID: 15959798.

66. Alomari MA, Welsch MA. Regional changes in reactive hyperemic blood flow during exercise training: time-course adaptations. Dyn Med. 2007; 6:1. PMID: 17222342.

67. McGowan CL, Levy AS, McCartney N, MacDonald MJ. Isometric handgrip training does not improve flow-mediated dilation in subjects with normal blood pressure. Clin Sci (Lond). 2007; 112:403–409. PMID: 17140398.

68. Patterson SD, Ferguson RA. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol. 2010; 108:1025–1033. PMID: 20012448.

69. Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011; 21:510–518. PMID: 21410544.

70. Iida H, Kurano M, Takano H, Kubota N, Morita T, Meguro K, Sato Y, Abe T, Yamazaki Y, Uno K, Takenaka K, Hirose K, Nakajima T. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol. 2007; 100:275–285. PMID: 17342543.

71. Credeur DP, Hollis BC, Welsch MA. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc. 2010; 42:1296–1302. PMID: 20019641.

72. Malfatto G, Facchini M, Sala L, Branzi G, Bragato R, Leonetti G. Effects of cardiac rehabilitation and beta-blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol. 1998; 81:834–840. PMID: 9555771.

73. Coats AJ. Exercise rehabilitation in chronic heart failure. J Am Coll Cardiol. 1993; 22(4 Suppl A):172A–177A.
74. Mayer F, Scharhag-Rosenberger F, Carlsohn A, Cassel M, Müller S, Scharhag J. The intensity and effects of strength training in the elderly. Dtsch Arztebl Int. 2011; 108:359–364. PMID: 21691559.

75. Heydari M, Boutcher YN, Boutcher SH. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin Auton Res. 2013; 23:57–65. PMID: 23104677.

76. Kiyohara Y. Trends in the type-specific incidence of stroke in a general Japanese population: the Hisayama Study. Nihon Rinsho. 2006; 64(Suppl 7):38–42.
77. Takamoto K, Sakai S, Hori E, Urakawa S, Umeno K, Ono T, Nishijo H. Compression on trigger points in the leg muscle increases parasympathetic nervous activity based on heart rate variability. J Physiol Sci. 2009; 59:191–197. PMID: 19340540.

78. Nakajima T, Iida H, Kurano M, Takano H, Morita T, Meguro K, Sato Y, Yamazaki Y, Kawashima S, Ohshima H, Tachibana S, Ishii N, Abe T. Hemodynamic responses to simulated weight-lessness of 24-h head-down bed rest and KAATSU blood flow restriction. Eur J Appl Physiol. 2008; 104:727–737. PMID: 18651162.

79. Figueroa A, Vicil F. Post-exercise aortic hemodynamic responses to low-intensity resistance exercise with and without vascular occlusion. Scand J Med Sci Sports. 2011; 21:431–436. PMID: 20136757.

80. Heffernan KS, Edwards DG, Rossow L, Jae SY, Fernhall B. External mechanical compression reduces regional arterial stiffness. Eur J Appl Physiol. 2007; 101:735–741. PMID: 17849144.

81. Burgomaster KA, Moore DR, Schofield LM, Phillips SM, Sale DG, Gibala MJ. Resistance training with vascular occlusion: metabolic adaptations in human muscle. Med Sci Sports Exerc. 2003; 35:1203–1208. PMID: 12840643.

82. Cook SB, Clark BC, Ploutz-Snyder LL. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc. 2007; 39:1708–1713. PMID: 17909396.

83. Fales JT, Heisey SR, Zierler KL. Blood flow from and oxygen uptake by muscle, during and after partial venous occlusion. Am J Physiol. 1962; 203:470–474. PMID: 13891428.

84. Karabulut M, Leal JA Jr, Garcia SD, Cavazos C, Bemben M. Tissue oxygenation, strength and lactate response to different blood flow restrictive pressures. Clin Physiol Funct Imaging. 2014; 34:263–269. PMID: 24119192.

85. Rossow LM, Fahs CA, Loenneke JP, Thiebaud RS, Sherk VD, Abe T, Bemben MG. Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin Physiol Funct Imaging. 2012; 32:331–337. PMID: 22856338.

Fig. 1
The impacts of BFR+LIRE on skeletal muscle and vasculature. Schematic of the impacts of BFR+LIRE on skeletal muscle (hormonal, and transcriptional factors) and vasculature (Endothelial, autonomic, hemodynamic function). GH, growth hormone; IGFs, insulin like growth factors; mTOR, mRNA in mammalan target of rapamycin; S6K1, ribosomal s6 kinase 1; PI3K, phosphoinositide 3- kinase; Akt, protein kinase B; VEGF, hemvascular endothelial growth factor; FMD, flow mediated dilation; ET-1, endothelin 1; (↑), increase; (↓), decrease; (-), no change; (?), inconsistent data.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download