Abstract
Cholecystokinin is known to be involved in the modulation of nociception and to reduce the efficacy of morphine analgesia. This study investigated the effects of intrathecal administration of morphine and the cholecystokinin type B antagonist CI-988 on below-level neuropathic pain after spinal cord injury in rats. We also examined the interaction of morphine and CI-988 in the antinociceptive effect. Both morphine and CI-988 given individually increased the paw withdrawal threshold to mechanical stimulation in a dose-dependent manner. The combination of ineffective doses of intrathecally administered CI-988 and morphine produced significant analgesic effects and the combination of effective doses resulted in analgesic effects that were greater than the sum of the individual effects of each drug. Thus, morphine showed a synergistic interaction with CI-988 for analgesia of central neuropathic pain.
Spinal cord injury (SCI) frequently leads to central neuropathic pain that is long-lasting and may cause a road block in the process of rehabilitation. Furthermore, neuropathic pain after SCI is usually refractory to conventional analgesic treatments and adequate control of central pain has remained a challenge [1,2]. The most potent analgesics, opioids, have been used for the alleviation of pain in clinical practice, but the neuropathic pain following SCI is known to be insensitive to opiate treatment [3,4]. In some cases central neuropathic pain may respond to opioid, but it often requires high doses that result in severe side effects [5]. Although there have been many research trials aiming to achieve effective therapeutic management or to develop novel agents for neuropathic pain, the results to date have shown limited success with serious accompanying side effects.
Cholecystokinin (CCK) is present in many areas of the central nervous system (CNS) and is known to reduce the antinociceptive efficacy of opioids [6,7]. Our previous report showed that expression of CCK mRNA, but not that of the CCK receptor, is increased after SCI using a spinal hemisection model [8]. Increased expression of CCK mRNA has also been reported in other types of experimental SCI models [6,7]. Previous reports, including studies from our laboratory, have demonstrated that neuropathic pain after SCI can be reduced by inhibition of CCK activity in rats [6,8,9]. Behavioral signs of pain upon mechanical stimulation were significantly reduced by systemic and intrathecal administration of the CCK blocker CI-988 [8].
Several studies have reported that CCK decreases the antinociceptive potency of morphine in rats with SCI [10,11]. In contrast, blocking CCK enhances the antinociceptive efficacy of morphine in different kinds of experimental pain models [9,10,12], indicating that CCK may modify opioid sensitivity in neuropathic pain states. In fact, the combination effects of morphine and CI-988 have already been reported in different types of pathologic pain conditions such as carrageenan-induced inflammation [13], peripheral nerve injury [7], and diabetic neuropathy [10]. However, there is no report on the role of the spinal CCK in opioid insensitivity for neuropathic pain after SCI due to lateral hemisection. Thus, the aim of this study was to characterize the interaction of morphine and CI-988, a highly selective CCK-B receptor antagonist, in the treatment of neuropathic pain following SCI.
All experimental procedures were performed in accordance with guidelines set by the Korea University College of Medicine Animals Research Policies Committee. Male Sprague-Dawley rats (N=80, weight 150~200 g at the time of operation) were used for this experiment. The animals were kept in a 12-hour light/12-hour dark cycle with light on at 7:00 A.M.
Under enflurane anesthesia (4% enflurane and 95% O2), a longitudinal incision was made exposing several levels of vertebrae, laminectomy was performed, and then the spinal cord was hemisected at T13 on the left side with a no. 11 blade scalpel. The wound was closed in anatomical layers, and the skin was closed with stainless steel wound clips.
Behavioral tests for motor function and hindlimb withdrawal threshold to mechanical stimulation were performed preoperatively and postoperatively. The tests were performed on each rat 1 day prior to surgery and 1, 4, 7, 14, and 21 days postoperatively (PO), and before the study of drug effects. Rats that showed a small degree of mechanical allodynia or contralateral hindlimb motor deficits were excluded.
Paw withdrawal threshold was assessed by measuring the threshold of brisk paw withdrawal response to graded mechanical stimulus with a series of von Frey filaments (0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50, and 15.10 g; Stoelting, Wood Dale, IL, USA). The rat was placed under a transparent plastic dome (28×10×10 cm) on a metal mesh floor and a von Frey filament was applied for 3~4 sec to the plantar surface of each hind paw while the filament was bent. The 50% withdrawal threshold was determined using the up-down method starting with the 2.0 g (4.31 mN) strength of filament, which is in the middle of the series of 8 von Frey filaments with logarithmically increasing stiffness (0.41~15.10 g). A withdrawal response led to presentation of the next weaker stimulus, and lack of withdrawal led to presentation of the next stronger stimulus. Stimuli were presented at intervals of several seconds. A brisk foot withdrawal to von Frey application was regarded as a positive response. Interpolation of the 50% threshold was carried out according to the method of Dixon [14].
To exclude the possibility that motor impairment might contribute to changes in withdrawal threshold during testing, a modification of the combined behavioral score (CBS) of Gale et al. [15] was performed at the time of the behavioral test. The CBS assigns a weight to each of the tests and combines them into one total score that represents the degree of motor impairment. The tests were motor scores, toe spread, righting reflex, extension withdrawal reflex, placing reflex, and inclined plane. Neurological function was evaluated by a scoring system that ranged from 0 for a normal rat to 90 for a completely paralyzed rat.
To determine the effect of intrathecally (IT) administered drugs on paw withdrawal threshold the rats were implanted with a catheter 3 weeks after hemisection. During this period motor function in hemisected rats recovered and the paw withdrawal threshold to mechanical stimulation decreased, as described in our previous report [8,16]. Under enflurane anesthesia (4% enflurane and 95% O2), the occipital muscles were separated from their attachment point and retracted caudally to expose the cisternal membrane at the base of the skull. Sterilized PE-10 tubing was threaded through an incision in the atlanto-occipital membrane to the 1 or 2 segments rostral to the hemisection site (5.5 cm). Animals showing evidence of neuromuscular dysfunction were excluded from further tests. After the experiment, the position of the IT catheter was confirmed by injection of Evans Blue dye after laminectomy.
Tests of drugs were performed 1 week after catheterization. The experimental design consisted of two components: (1) Evaluation of the separate effects of morphine and CI-988: morphine (1, 2, 5, or 10 µg) and CI-988 (100, 200, or 500 µg) were intrathecally injected to test their effects on paw withdrawal threshold following SCI and the 50% effective dose (ED50) of each drug was determined; (2) Evaluation of the CI-988-morphine interaction: the drugs were studied in combination at the ratio of ED50(CI-988):ED50(morphine). CI-988 and morphine were co-administered at different combined doses of CI-988: morphine (in µg) as follows: 97.1:2.9, 48.5:1.5, 19.4:0.6, and 9.7:0.3, which corresponded to total doses of 100, 50, 20, and 10 µg, respectively. Paw withdrawal threshold was measured 30 min before and 15, 30, 45, 60, and 90 min after injection of the drugs. The investigator conducting the behavioral tests was blinded to the injected drug. More than one test was performed on some rats. The interval between repeated tests was at least 3 days. Drugs were injected IT in a volume of 10 µl followed by 10 µl of saline to flush the catheter. CI-988 was a generous gift from Pfizer Inc. (Groton, CT, USA).
All values are expressed as mean±SEM. The withdrawal threshold data for the right and left hindpaws were combined. The Friedman repeated measures of analysis of variance followed by multiple comparison tests were used to compare behavioral test results before and after drug administration for the same animal. To compare the antinociceptive effect of drugs, the % maximal possible effect (MPE) was calculated using the following formula: % MPE=(post-drug response-baseline)/(maximal response-baseline) ×100, where the maximal response was 15 g. The ED50 was defined as the dose of drug that produced 50% of the maximal effect, and the 95% confidence limit and the SE were calculated.
Isobolographic analysis was performed as described previously [17,18]. First, the potency of the individual drugs was determined. The ED50(CI-988) was plotted on the abscissa and the ED50(morphine) on the ordinate. A theoretical simple additive line for a combination of the two drugs was generated by connecting the ED50 for CI-988 with that of morphine. The ED50(mix) for the combination of CI-988 and morphine was calculated by linear regression of the dose-response curve.
The potency and 95% confidence limit of the two drugs were compared using a t-test with the theoretical additive value (ED50(add)) obtained from the formula ED50(add)=ED50(CI-988)/(p1+Rp2), where R is the potency ratio of CI-988 to morphine, p1 is the proportion of CI-988 in the total dose, and p2 is the proportion of morphine in the total dose. The Mann-Whitney U test was used to compare the effect of morphine at different time points. p values less than 0.05 were considered significant.
Rats with SCI showed a significant decrease in paw withdrawal threshold to von Frey stimulation with maximal motor recovery 3 weeks after spinal hemisection, as in our previous report [19]. After implantation of the IT catheter the rats did not show impairment of motor function and maintained a decreased paw withdrawal threshold, which was interpreted as a behavioral sign of below-level neuropathic pain. The effects of IT morphine on the hindlimb withdrawal threshold to mechanical stimulation applied to the plantar surface of the both feet are shown in Fig. 1A. All doses of IT morphine tested in this study except for 1 µg significantly and dose-dependently increased the withdrawal threshold, as in our previous report [16]. Morphine at 10 µg produced a small increase in CBS in some rats, but overall this was not significantly different from the value obtained before morphine injection (data not shown). This antinociceptive effect of significantly increased withdrawal threshold lasted over a period from 15 to 90 min after injection of each dose of IT morphine. The effect of a higher concentration of morphine (10 µg, n=17) peaked at 30~45 min after injection. Saline had no effect on the responsiveness to mechanical stimuli with von Frey stimulation. The efficacy of IT morphine in increasing the withdrawal threshold to mechanical stimulation of both hindpaws is shown in Fig. 1B. The maximal possible effect at a dose of 10 µg morphine was 75.40±5.77%. The ED50 value of IT morphine for increasing the withdrawal threshold to von Frey stimulation following spinal cord hemisection was 6.59 µg.
IT administration of CI-988 (100, 200, or 500 µg) significantly increased withdrawal threshold to mechanical stimulation applied to the plantar surface of foot in a dose-dependent manner (Fig. 2A) without any change in motor function tested using CBS (data not shown). This result was similar to that of our previous report [8]. During this test time, the maximal increase in withdrawal threshold by higher doses of IT CI-988 (200 and 500 µg) was observed 15~30 min after injection, and then gradually diminished but persisted for 90 min, whereas the increased withdrawal threshold induced by the lower dose of CI-988 (100 µg, n=21) lasted for 60 min after injection. Saline treatment as a control (n=8) did not change the withdrawal threshold throughout the test period. The efficacy of IT CI-988 in increasing withdrawal threshold to mechanical stimulation of the feet is shown in Fig. 2B. The maximal effect at 500 µg CI-988 was 76.17±5.13%. The ED50 value of IT CI-988 for reducing the mechanical allodynia following spinal cord hemisection was 217.75 µg.
Since the ED50 values of IT CI-988 and IT morphine were 217.75 µg and 6.59 µg, respectively, the combined dose of CI-988 and morphine was selected at a fixed ratio of 33.05 (ED50(CI-988)/ED50(morphine)=217.75/6.59). The combined doses of CI-988:morphine used (97.1:2.9, 48.5:1.5, 19.4:0.6, and 9.7:0.3) corresponded to total doses of 100, 50, 20, and 10 µg. Morphine was injected 15 min before injection of CI-988 because of differences in the latency to peak effects between CI-988 and morphine, which occurred 15 and 30 min after injection, respectively. All combinations of IT CI-988 and IT morphine significantly increased the withdrawal threshold to mechanical stimulation applied to the plantar surface of foot compared to pre-drug values (Fig. 3). The maximal increase in withdrawal threshold was observed 15 min after injection. With the lowest doses, paw withdrawal threshold significantly increased from 15 min to 45 min after injection. The mixed 100-µg dose of CI-988 and morphine (97.1:2.9) used in this study significantly increased the withdrawal threshold from 1.78±0.16 g to 8.84±1.03 g, compared with changes from 1.90±0.13 g to 3.85±0.63 g for 2 µg morphine, 1.72±0.14 g to 6.90±1.01 g for 5 µg morphine, and 1.42±0.14 g to 5.72±0.79 g for 100 µg CI-988 administered alone.
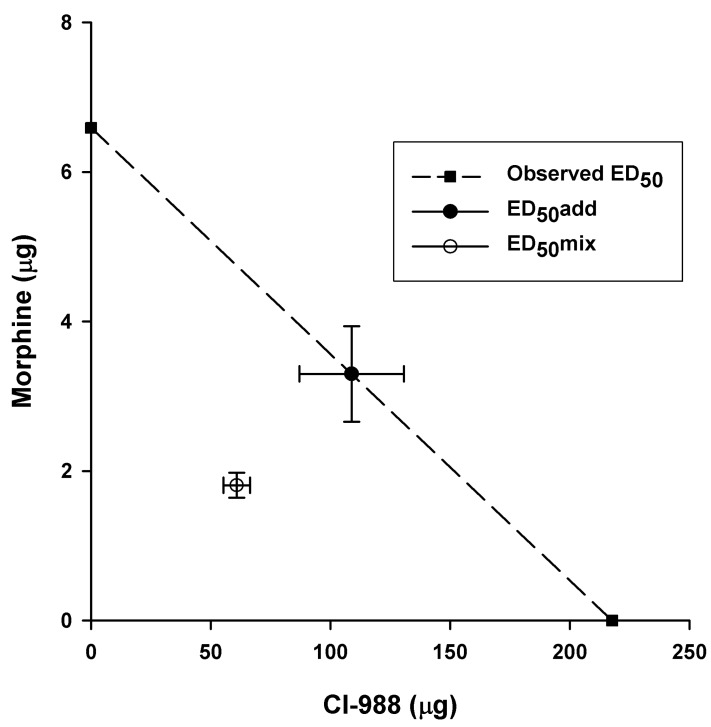
The ED50 of the combination of IT CI-988 plus IT morphine was 62.66 µg, plotted at (1.80, 60.86) on the morphine-CI-988 isobologram, whereas the theoretical additive ED50 for the combination CI-988 plus morphine was 112.17 µg plotted at (3.30, 108.87) (Fig. 4). The graphic illustration on the isobologram shows that the confidence intervals of these two points do not overlap, suggesting that the combination represents a superadditive interaction.
The present study demonstrates that blockade of spinal CCK with CI-988 enhances the analgesic efficacy of morphine in a model of central neuropathic pain following spinal cord hemisection. Intrathecal CI-988 increased the analgesic effects of morphine and intrathecal co-administration of CI-988 and morphine significantly increased paw withdrawal threshold following SCI. Furthermore, the analgesic effect of the combination of effective doses of CI-988 and morphine was greater than the sum of the individual effects of each drug. Our results were consistent with previous studies showing that CI-988 strongly potentiates the analgesic effect of morphine on neuropathic pain [9,10,11].
Intrathecally injected morphine and CI-988 significantly increased the paw withdrawal threshold in this study. Although there were no significant motor changes for all doses of IT CI-988 tested in the present study, slight motor depression was observed at the highest dose (10 µg) of IT morphine. The doses of morphine (1, 2, 5, and 10 µg) and CI-988 (100, 200, and 500 µg) used were chosen based on our previous study, in which these doses of each drug did not produce significant motor changes [8,16]. The finding that application of morphine and CI-988 at lesion sites reduced neuropathic pain suggests that the lesion site could be a major target area for modulating pain transmission after SCI.
It is well known that neuropathic pain is insensitive to opioids, including morphine. Two possible mechanisms have been proposed to explain the loss of opioid efficacy on neuropathic pain: (1) Damage to the nervous system leads to decreased expression of mu-opioid receptors in the spinal cord [20]; (2) Increased levels of anti-opioid peptides such as CCK in the spinal cord [21,22,23]. CCK is an anti-opioid substance that is localized in areas involved in pain perception at supraspinal (periaqueductal gray, thalamus, and cortex) and spinal (dorsal horn) levels [24]. CCK might be released at the spinal level by the descending facilitatory pathways after injury and mediate pain via inhibition of the endogenous opioid system [25]. Earlier studies showed that the level of CCK increases after injuries to the spinal cord or peripheral nerves [6,8,23,26] and that CCK is involved in pain modulation through its activity as an anti-opioid peptide [27,28]. Activation of the endogenous CCK system after SCI may explain why morphine has a reduced effect on central pain as endogenous CCK may also antagonize exogenously administered opioids.
There is some evidence supporting the notion that CCK attenuates endogenous opioidergic pain control and reduces the analgesic effect of exogenous opioid [10,11,29]. Xu et al. [9] showed that CI-988 strongly potentiated the effects of morphine in spinally injured rats. In the present study, the low intrathecal doses of 100 µg CI-988 and 2 µg morphine produced only a slight analgesic effect because the ED50 values of IT CI-988 and IT morphine were 217.75 µg and 6.59 µg, respectively. However, the lower combined dose induced a prominent analgesic effect compared to that expected from simple additional effects of the two drugs. These findings suggest that spinal CCK decreases opioid analgesia in a chronic pain state after SCI. The phenomenon whereby blocking CCK increases opioid efficacy is not restricted to neuropathic pain following SCI, but has been reported in different types of pathological pain conditions including carrageenan-induced inflammation [30], peripheral nerve injury [7], and diabetic neuropathy [10]. However, the interaction of CCK and opioids in the spinal level and the possible regulatory function of CCK in the efficacy of opioids for central pain following SCI are less well explored.
In this study, the combination of singly ineffective doses of intrathecally administered CI-988 and morphine produced significant analgesic effects and, moreover, the combination of effective doses resulted in analgesic effects that were greater than the sum of the individual effects of each drug. This superaddictive effect was confirmed by an isobolographic analysis. This result is similar to that of a previous study by Xu et al. [9] in which CI-988 strongly potentiated the analgesic effects of morphine in ischemic SCI rats. On the other hand, it has been reported that co-administration of morphine and CI-988 produces different interactions depending on the nature of the pain [10]. For example, co-administration of CI-988 and opioid drug failed to elicit a significantly potentiated analgesic effect on neuropathic pain after sciatic nerve injury [10], whereas the same combination of drugs produced significant superadditive analgesic effect on neuropathic pain in experimental diabetes [10]. It is possible that the disparity in the effects of a combination of morphine and CI-988 on neuropathic pain reflects differences in CCK activation in different pain conditions.
As CCK is considered to be an important endogenous control for spinal opioid activity, CCKergic antagonists may have high clinical potential in the development of new therapeutic agents. Especially, concomitant use of opioid and non-opioid drugs, each at low dosage, offers the possibility of optimal effect for pain control while minimizing side effects. Because of the inefficacy and dose-related side effects of single analgesic drugs, various combinations of multiple drugs have been tested to achieve effective pain management for chronic pain [31]. Recently developed heterobivalent compounds containing an opioid agonist and a CCK antagonist (RSA 504 and RSA 601) were reported to reduce behavioral signs of neuropathic pain following spinal nerve ligation [32].
The efficacy of CCK antagonists to reduce chronic neuropathic pain in human patients has also been tested [33]. However, unlike rodent studies, in which the CCK-B receptor seems to be important in regulating opioid activity, CCK-A receptors may be involved in pain modulation in humans. Proglumide, a non-specific CCK antagonist, reduced neuropathic pain [33] and enhanced the analgesic efficacy of morphine [34]. In contrast, L365-260, a specific CCK-B antagonist, did not augment the effect of morphine [35].
In summary, our results demonstrated the efficacy of a combination of CI-988 and morphine on below-level neuropathic pain following SCI. The fact that a combination of IT CI-988 and IT morphine showed synergistic analgesic effects confirms the interaction of the CCKergic system with opioids. The results suggest a possible clinical application of CI-988 or similar drugs in the management of chronic central pain. Furthermore, the superadditive effect of combined CI-988 and morphine provides a new strategy for treatment for this type of central neuropathic pain after SCI and may lead to new pharmacological therapeutic agents that achieve optimal pain control while minimizing side effects.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (MEST) 2011-005415.
References
1. Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000; 91:1493–1498. PMID: 11094007.

2. Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009; 60:202–213. PMID: 19154757.

3. Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990; 43:273–286. PMID: 1705692.

4. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010; 150:573–581. PMID: 20705215.

5. Rowbotham MC, Hansson PT, Fields HL, Hill RG, Marchettini P. Efficacy of opioids. In : Hansson PT, editor. Neuropathic pain: Pathophysiology and treatment. Seattle: ISAP Press;2001. p. 203–213.
6. Brewer KL, McMillan D, Nolan T, Shum K. Cortical changes in cholecystokinin mRNA are related to spontaneous pain behaviors following excitotoxic spinal cord injury in the rat. Brain Res Mol Brain Res. 2003; 118:171–174. PMID: 14559369.

7. Xu XJ, Puke MJ, Verge VM, Wiesenfeld-Hallin Z, Hughes J, Hökfelt T. Up-regulation of cholecystokinin in primary sensory neurons is associated with morphine insensitivity in experimental neuropathic pain in the rat. Neurosci Lett. 1993; 152:129–132. PMID: 8515864.

8. Kim J, Kim JH, Kim Y, Cho HY, Hong SK, Yoon YW. Role of spinal cholecystokinin in neuropathic pain after spinal cord hemisection in rats. Neurosci Lett. 2009; 462:303–307. PMID: 19619609.

9. Xu XJ, Hao JX, Seiger A, Hughes J, Hökfelt T, Wiesenfeld-Hallin Z. Chronic pain-related behaviors in spinally injured rats: evidence for functional alterations of the endogenous cholecystokinin and opioid systems. Pain. 1994; 56:271–277. PMID: 7912821.
10. Coudoré-Civiale MA, Courteix C, Fialip J, Boucher M, Eschalier A. Spinal effect of the cholecystokinin-B receptor antagonist CI-988 on hyperalgesia, allodynia and morphine-induced analgesia in diabetic and mononeuropathic rats. Pain. 2000; 88:15–22. PMID: 11098095.

11. Torres-López JE, Juárez-Rojop IE, Granados-Soto V, Diaz-Zagoya JC, Flores-Murrieta FJ, Ortíz-López JU, Cruz-Vera J. Peripheral participation of cholecystokinin in the morphine-induced peripheral antinociceptive effect in non-diabetic and diabetic rats. Neuropharmacology. 2007; 52:788–795. PMID: 17157334.

12. Idänpään-Heikkilä JJ, Perrot S, Guilbaud G, Kayser V. In mononeuropathic rats, the enhancement of morphine antinociception by L-365,260, a selective CCK(B) receptor antagonist, depends on the dose of systemic morphine and stimulus characteristics. Eur J Pharmacol. 1997; 325:155–164. PMID: 9163562.

13. Agnes RS, Ying J, Kövér KE, Lee YS, Davis P, Ma SW, Badghisi H, Porreca F, Lai J, Hruby VJ. Structure-activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides. 2008; 29:1413–1423. PMID: 18502541.

14. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.

15. Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985; 88:123–134. PMID: 3979506.

16. Kim J, Jung JI, Na HS, Hong SK, Yoon YW. Effects of morphine on mechanical allodynia in a rat model of central neuropathic pain. Neuroreport. 2003; 14:1017–1020. PMID: 12802194.

17. Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001; 298:865–872. PMID: 11504778.
18. Tallarida RJ, Stone DJ Jr, Raffa RB. Efficient designs for studying synergistic drug combinations. Life Sci. 1997; 61:PL 417–PL 425.

19. Kim J, Yoon YW, Hong SK, Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: time courses and their correlates. Neurosci Lett. 2003; 343:200–204. PMID: 12770696.

20. Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005; 117:77–87. PMID: 16098668.
21. Hebb AL, Poulin JF, Roach SP, Zacharko RM, Drolet G. Cholecystokinin and endogenous opioid peptides: interactive influence on pain, cognition, and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2005; 29:1225–1238. PMID: 16242828.

22. Wu HE, Schwasinger ET, Hong JS, Tseng LF. Pretreatment with antiserum against dynorphin, substance P, or cholecystokinin enhances the morphine-produced anti-allodynia in the sciatic nerve ligated mice. Neurosci Lett. 2005; 386:46–51. PMID: 15982809.

23. Xu XJ, Alster P, Wu WP, Hao JX, Wiesenfeld-Hallin Z. Increased level of cholecystokinin in cerebrospinal fluid is associated with chronic pain-like behavior in spinally injured rats. Peptides. 2001; 22:1305–1308. PMID: 11457525.

24. Wiesenfeld-Hallin Z, Xu XJ. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regul Pept. 1996; 65:23–28. PMID: 8876032.

25. Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000; 87:265–273. PMID: 10963906.

26. Verge VM, Wiesenfeld-Hallin Z, Hökfelt T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey. Eur J Neurosci. 1993; 5:240–250. PMID: 8261105.
27. Cesselin F. Opioid and anti-opioid peptides. Fundam Clin Pharmacol. 1995; 9:409–433. PMID: 8617406.

28. Mollereau C, Roumy M, Zajac JM. Opioid-modulating peptides: mechanisms of action. Curr Top Med Chem. 2005; 5:341–355. PMID: 15857316.

29. Zhou Y, Sun YH, Zhang ZW, Han JS. Increased release of immunoreactive cholecystokinin octapeptide by morphine and potentiation of mu-opioid analgesia by CCKB receptor antagonist L-365,260 in rat spinal cord. Eur J Pharmacol. 1993; 234:147–154. PMID: 8387008.
30. Stanfa LC, Dickenson AH. Cholecystokinin as a factor in the enhanced potency of spinal morphine following carrageenin inflammation. Br J Pharmacol. 1993; 108:967–973. PMID: 8485635.

31. Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012; 7:CD008943. PMID: 22786518.

32. Hanlon KE, Herman DS, Agnes RS, Largent-Milnes TM, Kumarasinghe IR, Ma SW, Guo W, Lee YS, Ossipov MH, Hruby VJ, Lai J, Porreca F, Vanderah TW. Novel peptide ligands with dual acting pharmacophores designed for the pathophysiology of neuropathic pain. Brain Res. 2011; 1395:1–11. PMID: 21550594.

33. McCleane G. The cholecystokinin antagonist proglumide has an analgesic effect when used alone in human neuropathic pain: A case report. Pain Clinic. 2003; 15:71–73.

34. McCleane GJ. The cholecystokinin antagonist proglumide enhances the analgesic efficacy of morphine in humans with chronic benign pain. Anesth Analg. 1998; 87:1117–1120. PMID: 9806692.

35. McCleane GJ. A randomised, double blind, placebo controlled crossover study of the cholecystokinin 2 antagonist L-365,260 as an adjunct to strong opioids in chronic human neuropathic pain. Neurosci Lett. 2003; 338:151–154. PMID: 12566175.

Fig. 1
Decreased paw withdrawal thresholds in SCI rats were significantly increased by IT morphine. (A) Effects of IT morphine on withdrawal threshold to mechanical stimulation applied to the plantar surface of the foot. Asterisks indicate significant difference from the pre-injection control value (PRE) (p<0.05). (B) Analgesic dose-response curve for morphine plotted at the time of peak effect (30 min). Results are expressed as percent (%) of the maximal effect.
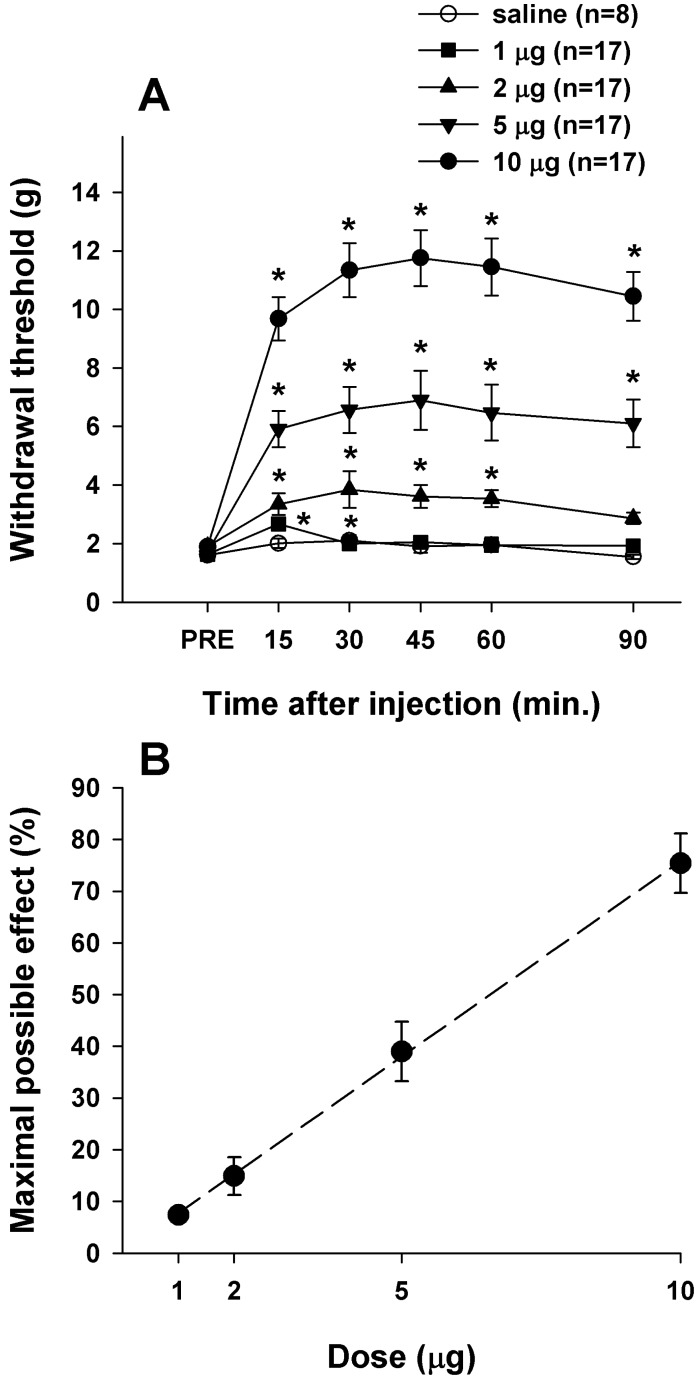
Fig. 2
Decreased paw withdrawal thresholds in SCI rats were significantly increased by IT CI-988. (A) Time course of the effect of IT CI-988 on withdrawal threshold to mechanical stimulation applied to the plantar surface of the foot. Asterisks indicate significant difference from the pre-injection control value (PRE). (B) Analgesic dose-response curve for CI-988 plotted at the time of peak effect (15 min). Results are expressed as percent (%) of the maximal effect.
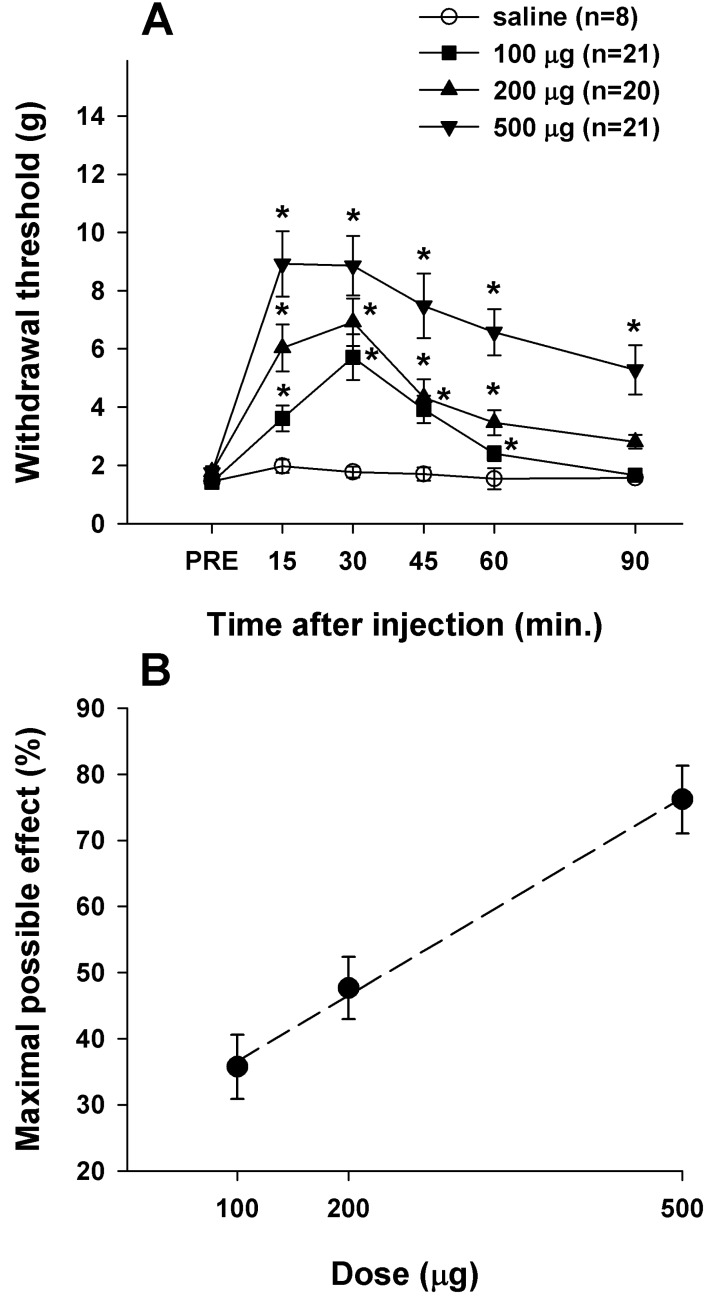
Fig. 3
The combination of CI-988 and morphine produced analgesic effects that were greater than the sum of the individual effects of each drug. (A) Time course of the effect of the combination of CI-988 and morphine on withdrawal threshold to mechanical stimulation applied to the plantar surface of the foot. The treatments administered were a combination of CI-988 and morphine at the following doses: CI-988:morphine ratios (in µg) of 97.1:2.9, 48.5:1.5, 19.4:0.6, and 9.7:0.3. Asterisks indicate significant difference from the pre-injection control value (PRE). (B) Analgesic dose-response curve for a combination of CI-988 and morphine plotted at the time of peak effect (15 min). Results are expressed as percent (%) of the maximal effect.
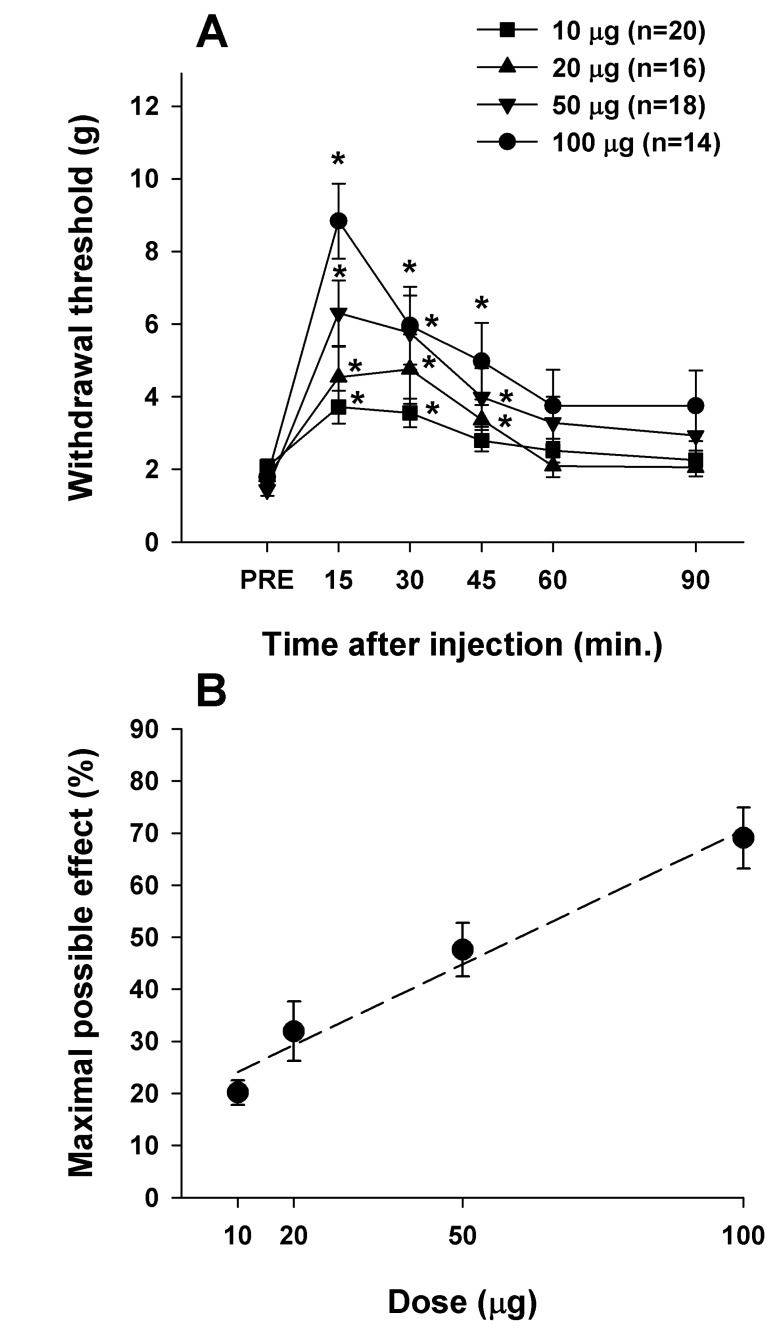
Fig. 4
Isobologram showing the effects of a combination of CI-988 and morphine on paw withdrawal threshold to mechanical stimulation. The dashed line represents the theoretical additive interaction. The interception of the dashed line on the ordinate and abscissa shows the observed ED50 values for morphine and CI-988 alone, respectively. The solid symbol represents the additive ED50(add) for the combination of CI-988 and morphine (97.1:2.9%). The actual ED50(mix) is represented by the open symbol. The standard errors for CI-988 and morphine are resolved into the corresponding components.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download