Abstract
DA-6034, a eupatilin derivative of flavonoid, has shown potent effects on the protection of gastric mucosa and induced the increases in fluid and glycoprotein secretion in human and rat corneal and conjunctival cells, suggesting that it might be considered as a drug for the treatment of dry eye. However, whether DA-6034 induces Ca2+ signaling and its underlying mechanism in epithelial cells are not known. In the present study, we investigated the mechanism for actions of DA-6034 in Ca2+ signaling pathways of the epithelial cells (conjunctival and corneal cells) from human donor eyes and mouse salivary gland epithelial cells. DA-6034 activated Ca2+-activated Cl- channels (CaCCs) and increased intracellular calcium concentrations ([Ca2+]i) in primary cultured human conjunctival cells. DA-6034 also increased [Ca2+]i in mouse salivary gland cells and human corneal epithelial cells. [Ca2+]i increase of DA-6034 was dependent on the Ca2+ entry from extracellular and Ca2+ release from internal Ca2+ stores. Interestingly, these effects of DA-6034 were related to ryanodine receptors (RyRs) but not phospholipase C/inositol 1,4,5-triphosphate (IP3) pathway and lysosomal Ca2+ stores. These results suggest that DA-6034 induces Ca2+ signaling via extracellular Ca2+ entry and RyRs-sensitive Ca2+ release from internal Ca2+ stores in epithelial cells.
Calcium (Ca2+) is the universal and pluripotent cellular signaling molecules that control widely cellular processes. Cytosolic Ca2+ signals are essential for the control of fluid and enzyme secretion from exocrine glands. Ca2+ signaling of these cells are initiated by Ca2+ release from intracellular stores, inositol 1,4,5-triphosphate (IP3) receptors (IP3Rs) and/or ryanodine receptors (RyRs) on the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) and the mitochondria. After rapid global Ca2+ signals, the clearance of Ca2+ from the cytoplasm is accomplished by activities of Ca2+- ATPases localized to both the ER (SERCAs) and plasma membrane (PMCAs) and then it is contributed substantially to the deactivation of chloride currents by SERCA [1].
The secretion can be significantly enhanced when both Ca2+ and cAMP signaling systems are activated, although the activation of Ca2+-dependent ion channels is the primary mechanism. The secretion from salivary glands is typically a watery fluid containing electrolytes and complex mixture of proteins, however mucous gland secretions are often quite viscous because of the discharge of large molecular weight mucins [2]. Tears are also composed of mucins, lipids, proteins, electrolytes and various other metabolites which are involved in various functions like ocular surface wound [3]. The secretion dysfunctions induce the oral pain, increased dental caries, and infections by opportunistic microorganisms in salivary glands, parotid and submandibular glands (SMG), and cause blurred and fluctuating vision and discomfort in lacrimal gland. These glands dysfunction are most frequently observed in diseases such as cystic fibrosis, or Sjögren's syndrome [2,4]. Patients with Sjögren's syndrome suffer from a severe deficit in fluid production by salivary glands and other exocrine organs.
Flavonoids are polyphenols found in a wide variety of medical plants and are known to have anti-inflammatory activities and selective inhibitors of lipoxygenase activity [5,6,7]. DA-6034 (7-carboxymethyloxy-3',4',5-trimethoxy flavones monohydrate) is a synthetic derivative of eupatilin, a flavonoid derivate. Eupatilin is a main component of the extract of Artermisiae species, a Korean folk medicine used in the treatment of chronic diarrhea. Eupatilin has also the pharmacological activities including the induction of apoptosis in both human gastric cancer cells and promyelocytic leukemia cells, and cell cycle arrest in ras-transformed human mammary epithelial cells [8,9]. DA-6034 has anti-inflammatory action through NF-κB down-regulation in gastric epithelial cells [10,11,12]. DA-6034 prevents gastric mucosal injury through increases of endogenous prostaglandin E2 (PGE2) synthesis and gastric mucus secretion [8]. Recently, several reports showed the therapeutic possibility for effects of DA-6034 such as increases of mucin and lacrimal secretions, and down-regulation of mitogen-activated protein kinase (MAPK) signaling in the dry eye model [13,14,15]. It was suggested that DA-6034 can relate the mechanisms of the Ca2+-activated fluid secretion in epithelial cells. However, whether DA-6034 induces Ca2+ signaling and its underlying mechanism in epithelial cells are not known. In this study, we aimed to investigate the physiological role of DA-6034 in Ca2+ signaling of various epithelial cells.
ICR strain mice (6~8 weeks) were sacrificed by cervical dislocation under CO2 anesthesia. Cells were prepared from the submandibular glands, parotids, and pancreases of ICR mice by limited collagenase digestion as previously described [16]. Following isolation, the acinar cells were suspended in an extracellular physiological salt solution (PSS), the composition of which was follows (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, adjusted to pH 7.4 and 310 mOsm.
During preparation for cornea buttons, human conjunctival tissue was saved and donated by the eye bank. The entire conjunctiva was dissected around the 2-mm lateral to limbus of the cornea. Conjunctival cells were isolated using the following method. First, conjunctiva tissue was incubated in phosphate-buffered saline with 1.4 U dispase (Sigma, St. Louis, MO, USA) for 1 hour in a 37℃ incubator. Incubated tissues were scraped and washed by media. Isolated conjunctival cells were cultured with keratinocyte growth medium (KGM; Lonza Walkersville, Inc., Walkersville, MD, USA). For multilayer cell cultures, isolated conjunctival cells were cultured on 6-well plates containing Transwell permeable supports with KGM first. After the cells covered the bottom of Transwell plates, media was removed and replaced with differentiation media [1:1, KGM +0.12% bovine serum albumin (BSA), 10 nM retinoic acid (RA): low glucose Dulbecco's Minimal Essential Medium (DMEM)]. During cell differentiation, medium was replaced every day. Human corneal epithelial cell line (CEC) was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37℃ in humidified 5% CO2 in air. CEC cells were grown in bronchial epithelial cell growth medium (BEGM; Lonza Walkersville, Inc., Walkersville, MD, USA). BEGM consists of bronchial epithelial basal medium (BEBM) supplemented with recombinant human epidermal growth factor (rhEGF), bovine pituitary extract (BPE), insulin, hydrocortisone, transferrin, tri-iodothyronine, epinephrine, pituitary extract (PE), GA-1000, and retinoic acid. Cells were plated onto glass coverslips for 4 days or until 75% confluence before calcium imaging.
The method of perforated patch-clamp has been modified previously [17,18]. Briefly, Whole-cell voltage-clamp recordings were made using the perforated patch-clamp method at room temperature (22~25℃). Currents were recorded using a MultiClamp 700B amplifier (Axon Instruments, Union City, CA, USA), subsequently digitized with a sampling rate of 10 kHz, and analyzed using pCLAMP10 software (Axon Instruments). The pipette resistance varied between 3~5 MΩ. Whole-cell currents were elicited by voltage ramps from -100 mV to +100 mV (800-ms duration) applied every 10 s from a holding potential of -40 mV. Pipettes for recordings of Cl- currents were filled with an internal solution containing (in mM): 140 mM N-methyl-D-glucamine (NMDG)-Cl, 0.5 mM EGTA, 10 mM HEPES, 1 mM MgCl2 and 1 mM Mg-ATP, adjusted to pH 7.2 [18]. Nystatin was diluted to a final concentration of 250 µg/ml in the internal solution. The external solution containing (in mM): 140 mM NMDG-Cl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM glucose, adjusted to pH 7.4.
The method of [Ca2+]i imaging has been modified previously [19]. Briefly, cells were incubated with 2 µM Fluo-4/AM or Fura-2/AM and 0.05% Pluronic F-127 for 30 min in PSS at room temperature. Fluo-4/AM fluorescence was measured at 490 nm (fluorescence intensity=F/F0) using a Molecular Devices (Downingtown, PA, USA) imaging system. The emitted fluorescence was monitored with a charge-coupled device camera (Photometrics, Tucson, AZ, USA) attached to an inverted microscope. Fura-2 fluorescence intensity also was measured using excitation wave lengths of 340 and 380 nm, and emitted fluorescence 510 nm (Ratio=F340/F380). Fluorescence images were obtained at 2-s intervals. All data were analyzed using MetaFlour software (Molecular Devices).
Fluo-4/AM and fura-2/AM were purchased from Invitrogen Molecular Probes (Eugene, OR, USA). SFK96365 hydrochloride, ionomycin, cyclopiazonic acid (CPA), U73122, U73343, ryanodine, ruthenium red, and 2-aminoethoxydiphenyl borate (2APB) were from Tocris Bioscience (Bristol, UK). Bafilomycin A1 was obtained from Alexis Biochemicals (San Diego, CA, USA). Collagenase P was purchased from Roche (Indianapolis, IN, USA). All other chemicals were purchased from Sigma. Stock solutions of all drugs were made in distilled water, except, 2APB (made in ethanol), and DA-6034, fluo-4/AM, fura-2/AM, ionomycin, CPA, SKF96365, U73122, U73343, and ryanodine (made in DMSO).
In the present study, we examined the effects of DA-6034 on Ca2+ signaling in primary cultured human conjunctival epithelial cells. We observed that DA-6034 (10 µM) stimulation activated Cl- transport across the Ca2+-activated Cl- channels (CaCCs) in conjunctival epithelial cells, when compared to control (Fig. 1A). Extracellular application of ATP (100 µM) evoked transient increases of [Ca2+]i (Fig. 1B). Likewise, 100 µM DA-6034 induced transient increases of [Ca2+]i in primary cultured human conjunctival epithelial cells (Fig. 1C). These results suggest that DA-6034 activate the CaCCs and induced transient [Ca2+]i increases in primary cultured human conjunctival epithelial cells.
To confirm similar effects of DA-6034 on [Ca2+]i in various epithelial cells, we performed experiments for [Ca2+]i increases by DA-6034 in salivary gland epithelial cells and human corneal epithelial cell line (CEC). Application of 100 µM DA-6034 induced an increase of [Ca2+]i in salivary gland (SMG and parotid) and pancreatic acinar cells (Fig. 2A~C). DA-6034 induced [Ca2+]i increase was also observed in CEC cells (Fig. 2D). The difference of DA-6034 induced [Ca2+]i increases was depending on cell types (Fig. 2E). These results suggest that DA-6034 induce the [Ca2+]i increases in various epithelial cells.
To determine sources of [Ca2+]i increased by DA-6034, the experiments with Ca2+-free environment or extracellular Ca2+ entry blockers were performed. First, DA-6034 induced [Ca2+]i increase was completely inhibited by Ca2+-free buffered solution in SMG and parotid acinar cells (Fig. 3A and B). We also observed these similar effects in CEC cells (data not shown). The [Ca2+]i increases by DA-6034 was also decreased by treatments with 100 µM La3+ and Gd3+, non-specific voltage-dependent Ca2+ channel blockers (Fig. 3Ca and 3Cb), and 30 µM SKF96365, receptor/store-operated Ca2+ channel blocker, and 100 µM 2APB, store-operated Ca2+ entry blocker in CEC cells (Fig. 3Cc and 3Cd). These results suggest that the DA-6034 induced [Ca2+]i increase is related to Ca2+ entry from the extracellular space.
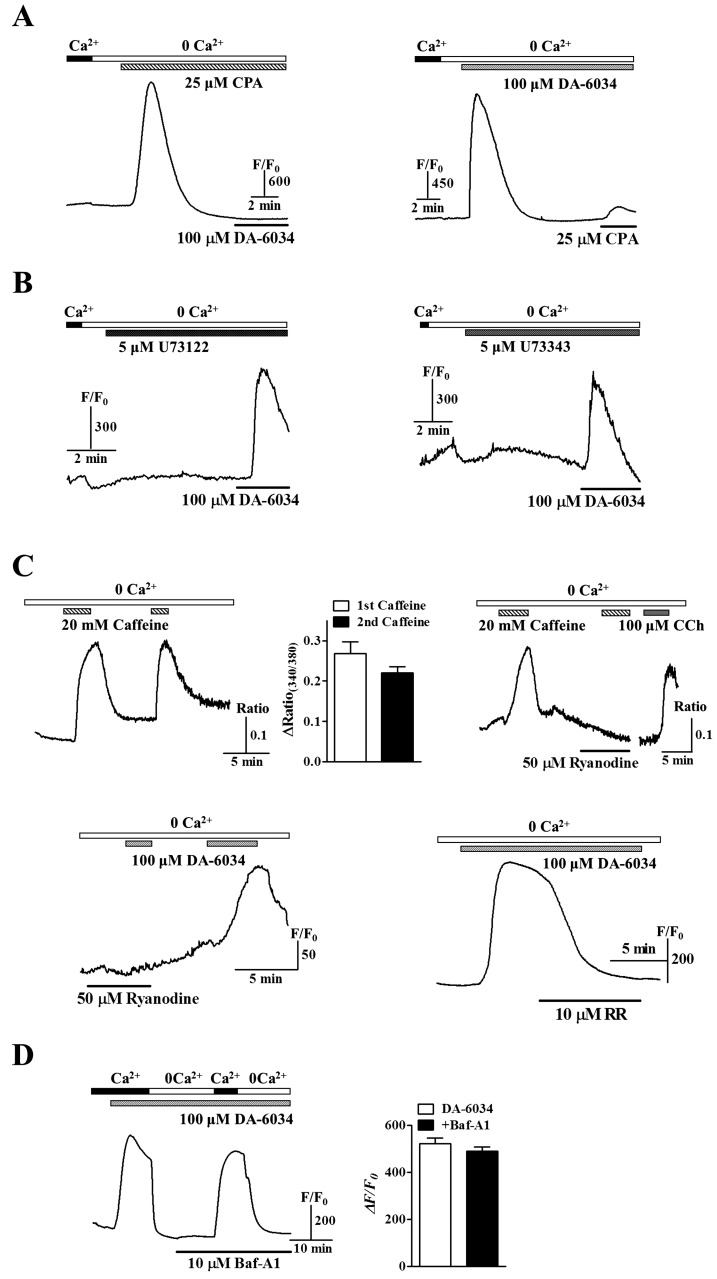
In Fig. 3, [Ca2+]i increases by DA-6034 also showed the possibility which DA-6034 induced [Ca2+]i increases are related to the intracellular Ca2+ stores. To investigate whether DA-6034-induced [Ca2+]i increases were related to Ca2+ release from internal stores, we used 25 µM CPA to deplete the ER and then applied DA-6034. In SMG acinar cells, treatment with CPA in nominally Ca2+-free buffered solution showed inhibitory effects for the [Ca2+]i increase by DA-6034 (Fig. 4A, left panel) and also disappeared the effect of ER Ca2+ depletion by CPA in order changing (Fig. 4A, right panel), suggesting that DA-6034 induced [Ca2+]i increases are related to Ca2+ release from the ER. Mostly ER Ca2+ release can be mediated through the IP3Rs [20]. Therefore, we examined the effects of DA-6034 in the treatment of PLC inhibitor U73122 whether DA-6034 induced [Ca2+]i increase is evoked by activation of PLC/IP3 pathway. The [Ca2+]i increase by DA-6034 still remained by the treatment of PLC inhibitor U73122 and U73343 (an inactive analog of U73122) in SMG acinar cells (Fig. 4B). These results suggest that effects of DA-6034 can be mediated by other intracellular Ca2+ stores, such as RyRs and lysosomal Ca2+ pools. Then, we confirmed the actions of RyRs using caffeine (an activator of RyRs), ryanodine (an inhibitor of RyRs), and ruthenium red (RR, an inhibitor of RyRs) (Fig. 4C, upper panels). Therefore, we showed inhibitory effects of DA-6034 induced [Ca2+]i increase by ryanodine and RR in SMG acinar cells (Fig. 4C, bottom panels). However, the [Ca2+]i increase by DA-6034 still remained by the treatment of bafilomycin A1 (Baf-A1, an inhibitor of vacuolar-type H+-ATPase) (Fig. 4D). Taken together, these results suggest that DA-6034 induced [Ca2+]i increase is dependent on RyRs but not the PLC/IP3 pathway and lysosomal Ca2+ pools.
In this study, we demonstrated for the first time the [Ca2+]i increases by DA-6034 through the regulation of extracellular Ca2+ entry and intracellular Ca2+ release in human conjunctival/corneal epithelial cells and mouse salivary gland epithelial cells. These effects could be the result of the released Ca2+ accelerating activity during the stimulated fluid secretion by DA-6034, which is the previous reported physiological function of the DA-6034 such as increases of mucus content and endogenous PGE2 synthesis in gastrointestinal tracts and tear secretion and mucin production in conjunctival goblet cells [8,13,14]. These results suggest that DA-6034 is a main regulator to modulate secretory activities in exocrine gland cells and also indicates possibilities to a DA-6034-mediated modulation on intracellular Ca2+ signaling in fluid secretion of exocrine gland [21]. Usually, the mucin and fluid secretion are stimulated predominantly by Ca2+ dependent pathways that are activated by extracellular ATP and other stimulators. Then, released peptides, such as secretin and VIP etc., stimulate adenylyl cyclase activities via G-protein coupled receptors, and subsequently increases of cAMP, PKA, and PKC induce secretions of mucin, fluid, and anion [2,20,21]. However, potential redox state of epithelial cells leads to oxidative stress in the bacteria infection or cystic fibrosis. The distribution of unbalanced redox can evoke inflammatory signaling pathway and apoptosis, such as NF-κB activation and inflammation [22,23]. DA-6034 suppressed iNOS induction, NF-κB activation, p65 nuclear translocation, and restored IκBα level in the cytoplasm and dissociates the IKK-γ-Hsp90 complex in gastric cancer models [11,12]. In addition, DA-6034 attenuated JNK and p38 MAPK and also inhibited NF-κB activation in dry eye model cells [15]. These results suggest that DA-6034 has anti-inflammatory and anti-apoptotic effects in epithelial cells. This is supported by previous reports showing that DA-6034 has increased mucin secretion and endogenous PGE2 synthesis which prevented gastric mucosal injury and dry eye [8,13,14].
We also examined the sources of [Ca2+]i increased by DA-6034. Our results demonstrated that the effects of DA-6034 could be dependent on extracellular and intracellular Ca2+ levels, however this [Ca2+]i mobilization was included the RyRs but not the PLC/IP3 pathway. The [Ca2+]i mobilization of DA-6034 through the RyRs is a novel finding in our results. Ca2+ mobilization is regarded as a key element in signal transduction and secretory process in exocrine cells [2,21]. In common with acinar cells from exocrine tissue, IP3Rs are mainly expressed the apical region and exhibit the initiation and rapid global Ca2+ signals. Ryanodine receptors (RyRs) also intracellular Ca2+ release channels that belong to a family related to IP3Rs. RyRs are predominantly localized basal regions. They are gated by elevations in cytoplasmic Ca2+ in a process termed calcium-induced calcium release (CICR) and contribute to the mechanisms of regenerative Ca2+ waves in a various cells. Several studies reported that it could be cAMP-induced Ca2+ release without elevation of IP3 through the activation of RyRs in rat parotid acinar cells or microsomal vesicles [2]. Several reports showed that RyRs (RyR type 1 and type 3)-mediated CICR regulated the corticotropin-relasing factor-induced adrenocorticotropin secretion in mouse corticotroph cell line [24] and puerarin (a traditional Chinese medicine) facilitated CICR via RyRs and cAMP/PKA signaling pathway in rat hippocampal neurons [25]. CaCCs are activated by both Ca2+ influx and CICR from internal stores. Treatments of caffeine activated CaCCs in DRG neurons [26]. In our results, DA-6034 activated CICR through RyRs and CaCCs in epithelial cells. Therefore, it suggests that activated [Ca2+]i increases by DA-6034 can mediate related responses between RyRs and CaCCs.
Taken together, DA-6034 could increase intracellular Ca2+ through RyRs and improve secretory process such as tear, mucin and salivary fluid in mouse salivary gland cells and human epithelial cells from eyes. Although further studies of mechanisms are necessary, these findings already suggest that DA-6034 may be a potential therapeutic agent for the dry eye and mouth diseases.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2012R1A2A1A01003487, 2007-0056419).
References
1. Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008; 70:273–299. PMID: 17850212.

2. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005; 67:445–469. PMID: 15709965.

3. Aluru SV, Agarwal S, Srinivasan B, Iyer GK, Rajappa SM, Tatu U, Padmanabhan P, Subramanian N, Narayanasamy A. Lacrimal proline rich 4 (LPRR4) protein in the tear fluid is a potential biomarker of dry eye syndrome. PLoS One. 2012; 7:e51979. PMID: 23272196.

4. Cheng EY, Goss CH, McKone EF, Galic V, Debley CK, Tonelli MR, Aitken ML. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros. 2006; 5:85–91. PMID: 16650742.

5. Ferrándiz ML, Alcaraz MJ. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 1991; 32:283–288. PMID: 1650522.

6. Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, Kang J, Wang JM. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000; 49:295–306. PMID: 10996027.

7. Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem Pharmacol. 2003; 65:773–781. PMID: 12628491.

8. Choi SM, Shin JH, Kang KK, Ahn BO, Yoo M. Gastroprotective effects of DA-6034, a new flavonoid derivative, in various gastric mucosal damage models. Dig Dis Sci. 2007; 52:3075–3080. PMID: 17406830.

9. Kim YS, Son M, Ko JI, Cho H, Yoo M, Kim WB, Song IS, Kim CY. Effect of DA-6034, a derivative of flavonoid, on experimental animal models of inflammatory bowel disease. Arch Pharm Res. 1999; 22:354–360. PMID: 10489873.

10. Kim EJ, Chung MY, Chung HJ, Son MW, Kwon JW, Yoo M, Lee MG. Pharmacokinetics of 7-carboxymethyloxy-3',4',5-trimethoxy flavone (DA-6034), a derivative of flavonoid, in mouse and rat models of chemically-induced inflammatory bowel disease. J Pharm Pharmacol. 2006; 58:27–35. PMID: 16393461.

11. Ko SH, Yoo DY, Kim YJ, Choi SM, Kang KK, Kim H, Kim N, Kim JS, Kim JM. A mechanism for the action of the compound DA-6034 on NF-κB pathway activation in Helicobacter pyloriinfected gastric epithelial cells. Scand J Immunol. 2011; 74:253–263. PMID: 21623862.

12. Lee JS, Kim HS, Hahm KB, Sohn MW, Yoo M, Johnson JA, Surh YJ. Inhibitory effects of 7-carboxymethyloxy-3',4',5-trimethoxyflavone (DA-6034) on Helicobacter pylori-induced NF-kappa B activation and iNOS expression in AGS cells. Ann N Y Acad Sci. 2007; 1095:527–535. PMID: 17404066.
13. Choi SM, Lee YG, Seo MJ, Kang KK, Ahn BO, Yoo M. Effects of DA-6034 on aqueous tear fluid secretion and conjunctival goblet cell proliferation. J Ocul Pharmacol Ther. 2009; 25:209–214. PMID: 19456255.

14. Choi SM, Seo MJ, Lee YG, Lee MJ, Jeon HJ, Kang KK, Ahn BO, Yoo M. Effects of DA-6034, a flavonoid derivative, on mucin-like glycoprotein and ocular surface integrity in a rabbit model. Arzneimittelforschung. 2009; 59:498–503. PMID: 19998577.

15. Seo MJ, Kim JM, Lee MJ, Sohn YS, Kang KK, Yoo M. The therapeutic effect of DA-6034 on ocular inflammation via suppression of MMP-9 and inflammatory cytokines and activation of the MAPK signaling pathway in an experimental dry eye model. Curr Eye Res. 2010; 35:165–175. PMID: 20136427.

16. Jo H, Byun HM, Lee SI, Shin DM. Initiation site of Ca2+ entry evoked by endoplasmic reticulum Ca2+ depletion in mouse parotid and pancreatic acinar cells. Yonsei Med J. 2007; 48:526–530. PMID: 17594163.
17. Yang YM, Jung HH, Lee SJ, Choi HJ, Kim MS, Shin DM. TRPM7 Is Essential for RANKL-Induced Osteoclastogenesis. Korean J Physiol Pharmacol. 2013; 17:65–71. PMID: 23440520.

18. Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997; 273:C442–C455. PMID: 9277342.

19. Park S, Lee SI, Shin DM. Role of regulators of g-protein signaling 4 in Ca2+ signaling in mouse pancreatic acinar cells. Korean J Physiol Pharmacol. 2011; 15:383–388. PMID: 22359476.
20. Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell. 2004; 96:3–17. PMID: 15093123.

21. Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012; 2:a009563. PMID: 22675668.

22. Haddad JJ. Redox regulation of pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear translocation and activation. Biochem Biophys Res Commun. 2002; 296:847–856. PMID: 12200125.
23. Rottner M, Freyssinet JM, Martínez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res. 2009; 10:23. PMID: 19284656.

24. Yamamori E, Iwasaki Y, Oki Y, Yoshida M, Asai M, Kambayashii M, Oiso Y, Nakashima N. Possible involvement of ryanodine receptor-mediated intracellular calcium release in the effect of corticotropin-releasing factor on adrenocorticotropin secretion. Endocrinology. 2004; 145:36–38. PMID: 14592949.

25. Lin F, Xin Y, Wang J, Ma L, Liu J, Liu C, Long L, Wang F, Jin Y, Zhou J, Chen J. Puerarin facilitates Ca2+-induced Ca2+ release triggered by KCl-depolarization in primary cultured rat hippocampal neurons. Eur J Pharmacol. 2007; 570:43–49. PMID: 17610871.
26. Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005; 67:719–758. PMID: 15709976.

Fig. 1
Cl- currents and [Ca2+]i after application of DA-6034 in primary cultured human conjunctival epithelial cells. (A) Cl- currents were activated by 10 µM DA-6034 in primary cultured human conjunctival epithelial cells. (B) Treatment with 100 µM ATP and (C) 100 µM DA-6034 induced increases of [Ca2+]i in human conjunctival cells (dotted cells in figure) using Fluo-4 florescence dye.
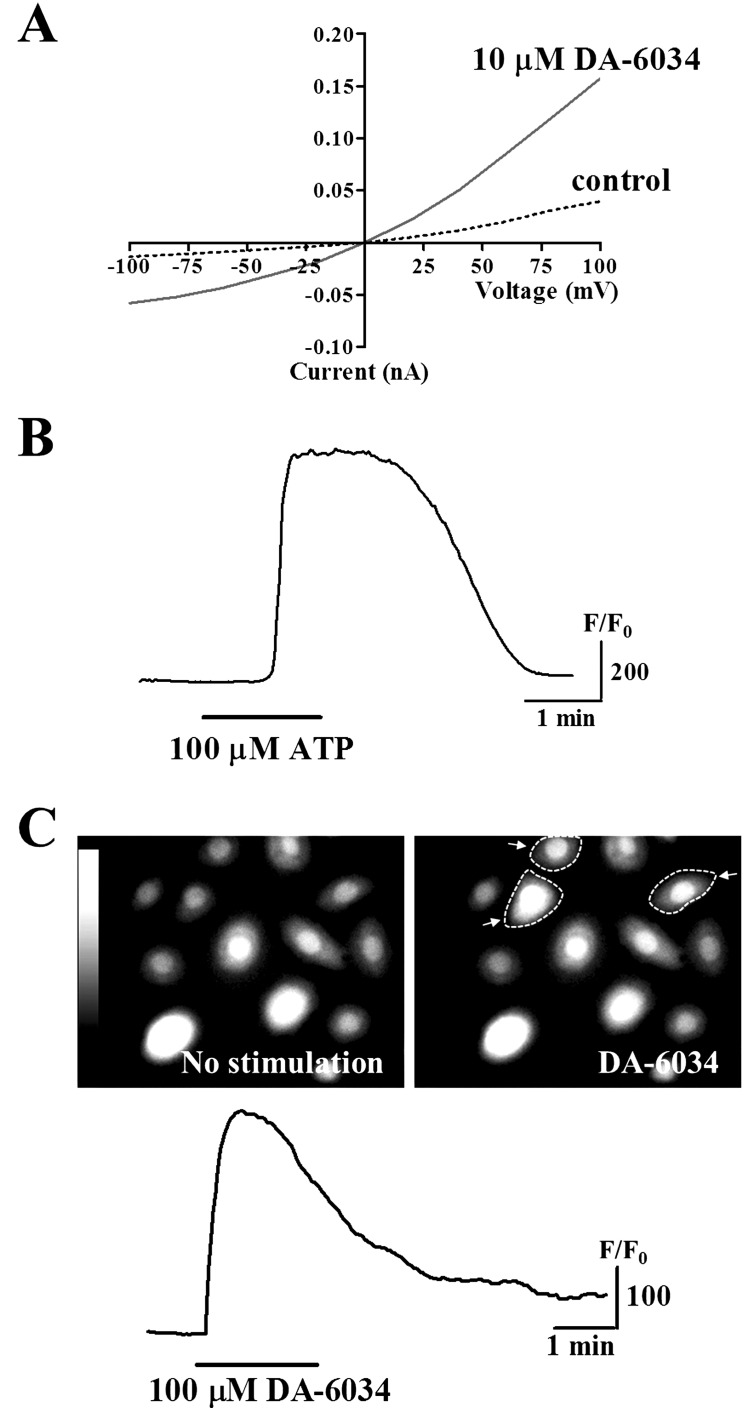
Fig. 2
Effects of [Ca2+]i increases by treatments of DA-6034 in cells. (A~C) Treatment of 100 µM DA-6034 induced increases of [Ca2+]i and also showed [Ca2+]i increases by application of 1 mM carbachol in salivary gland (SMG and parotid) and pancreatic acinar cells using Fluo-4 fluorescence dye. (D) CEC cells were also increased [Ca2+]i by treatments DA-6034 and 100 µM ATP (square box). (E) Bar graph presented the difference of [Ca2+]i increases by DA-6034 and carbachol/ATP in various cells. Data were expressed as the mean±SEM. **p<0.01, ***p<0.001 compared with carbachol or ATP. n.s., not significant.
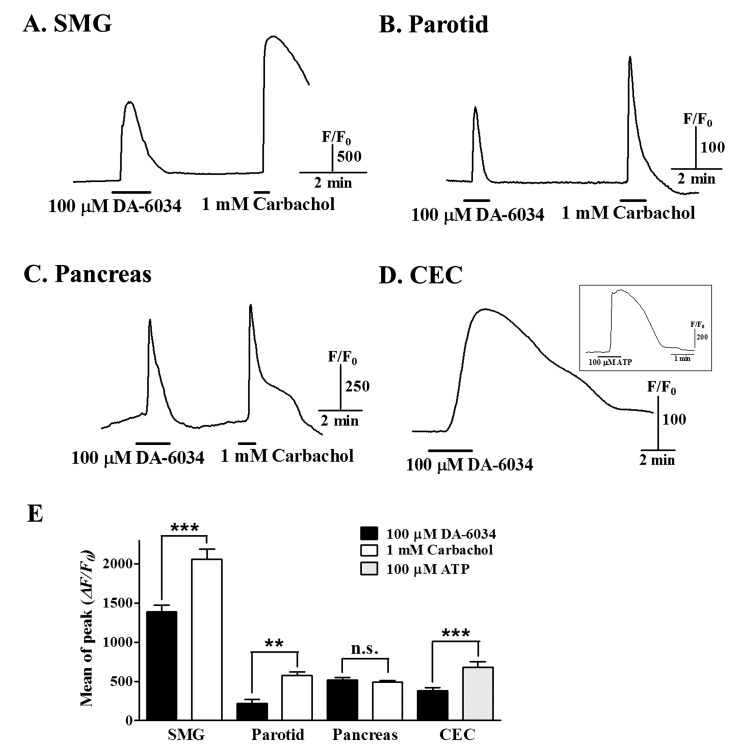
Fig. 3
Dependence on extracellular Ca2+ of DA-6034 induced [Ca2+]i increases. (A, B) The effects of [Ca2+]i increases by DA-6034 were inhibited by Ca2+-free solution in SMG and parotid acinar cells. (C) The effects of [Ca2+]i increases by DA-6034 were inhibited by 100 µM La3+ (a) and 100 µM Gd3+ (b), which block a wide range of Ca2+-permeable channels, in CEC cells. 30 µM SKF96365 (c) and 100 µM 2APB (d) also blocked DA-6034 induced [Ca2+]i increases through the inhibition of the receptor/store-mediated Ca2+ influx.
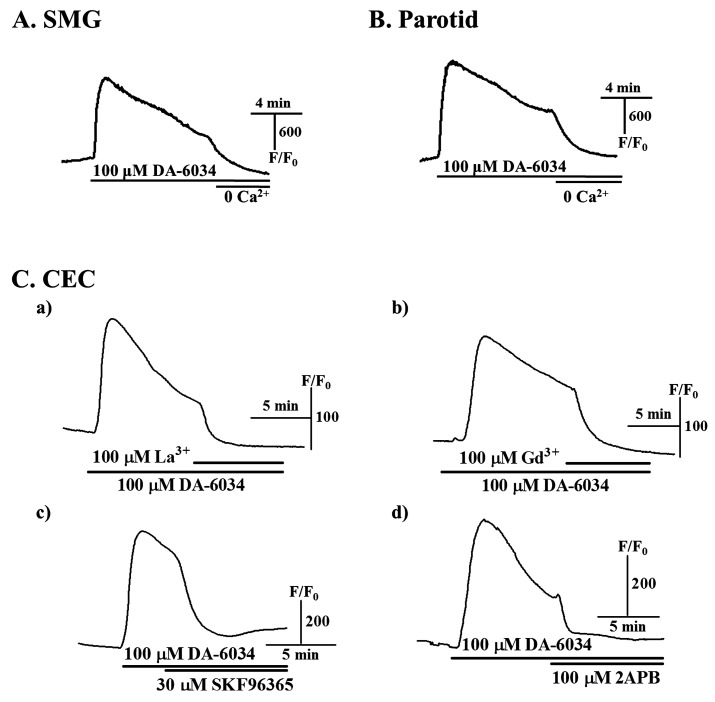
Fig. 4
Relationship with internal Ca2+ stores and DA-6034. (A) DA-6034 induced [Ca2+]i increases were disappeared after ER depletion by CPA. (B) However, DA-6034 induced Ca2+ influx not inhibited by the PLC inhibitor, U73122, and its close analogue, U73343. (C) Caffeine induced [Ca2+]i increases were repeated by the second application of caffeine (upper left panel) and were blocked by ryanodine, which was an inhibitor of ryanodine receptors (upper right panel). DA-6034 induced [Ca2+]i increases also inhibited by ryanodine (bottom left panel) and RR (bottom right panel). (D) DA-6034 induced [Ca2+]i increases were not inhibited by Baf-A1, which was a specific inhibitor of vacuolar-type H+-ATPase. Data were expressed as the mean±SEM.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download